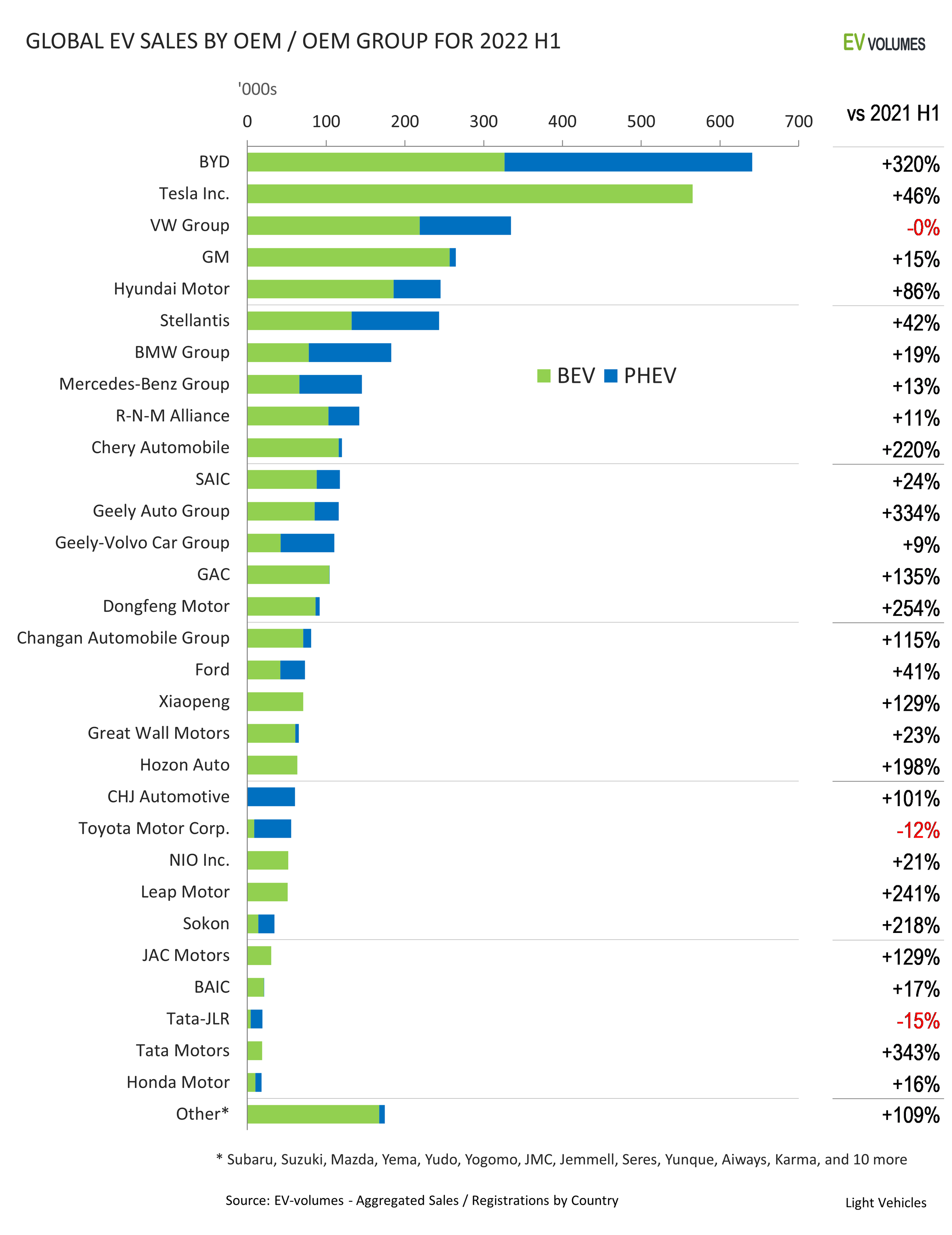
Headlines
The Top 5 graphite miners to watch in 2022 as battery materials’ prices rise
Originally posted on Investorintel.com
2021 has seen key electric vehicle (“EV”) and battery metals lithium, cobalt, nickel, and neodymium/praseodymium (NdPr) prices all rise strongly. But what about graphite?
It is starting to look like graphite will be next and 2022 will be graphite’s year. As reported on December 15, 2021 by Reuters:
“China EV, battery makers grapple with graphite squeeze……Chinese producers have their work cut out keeping up with global demand for graphite, which has surged along with rapid growth in the battery market in recent years…..Consultancy Benchmark Mineral Intelligence [BMI] sees a roughly 20,000 tonne graphite deficit in 2022, versus a similar-sized surplus last year…..Top global EV battery maker Contemporary Amperex Technology Co Ltd (CATL) (300750.SZ) is “desperate” to secure supply of key ingredients such as graphite to keep up with rising orders, said a person with knowledge of the matter.”
Top 5 graphite miners to watch in 2022 (in alphabetical order)
Leading Edge Materials Corp.
NextSource Materials Inc.
Syrah Resources Limited
Talga Group Ltd.
Triton Minerals Limited
Leading Edge Materials Corp. (TSXV: LEM | OTCQB: LEMIF)
Leading Edge Materials Corp. (“Leading Edge”) is a Canadian company focused on developing a portfolio of critical raw material projects located in the European Union.
Leading Edge 100% owns the producing Woxna Graphite mine and processing plant in Sweden. Woxna has a Total Resource estimate of 10.7 Mt Measured & Indicated @ 7.75% graphite plus 2.51 Mt Inferred @ 8.16% graphite.
Leading Edge plans to build a vertically integrated mine to anode material production capability, by producing coated spherical purified graphite anode material. The Company has completed a PEA (June 2021 PEA) on the mine to anode material project. The post-tax NPV8% is US$248 million with a post tax IRR of 37.4%, over a 15 year mine life. Initial CapEx is US$121 million. The 2021 PEA is based on 159,967 tpa of graphite production and 7,435 tpa of coated spherical purified graphite (“CSPG”) production. Leading Edge states: “Operating cost per tonne of coated spherical purified graphite (CSPG) of $2,519 after revenue credit from micronized graphite product vs forecasted selling price of $10,000 per tonne.”
Interestingly, Leading Edge 100% own the Norra Kärr REE Rare earths project (dysprosium, terbium, and neodymium/praseodymium (NdPr)) in Sweden which has a PEA completed. Plus they own 51% (option to increase to 90%) of the Bihor Sud Nickel Cobalt exploration stage project in Romania.
Leading Edge trades on a market cap of only C$56 million. Significant potential upside if they can succeed in their plans.
NextSource Materials Inc. (TSX: NEXT | OTCQB: NSRCF)
NextSource Materials Inc. (“NEXT”) is rapidly developing its 100% owned Molo Graphite Project in Madagascar. Financially boosted by serious investors and new Chairman Sir Mick Davis, NEXT’s Molo Graphite Project is fully funded to stage 1 production. The Project is designed with a modular approach in mind with the first stage production target of 17,000 tpa of flake graphite. Stage 1 construction is underway with mine commissioning expected in Q2 2022. NEXT has a 10 year 35,000 tpa binding-offtake deal with Thyssenkrupp Materials Trading.
Stage 2 expansion is undergoing a Technical study to assess a production capacity of at least 150,000 tpa.
NEXT is also working on a three-way collaboration to build a Battery Anode Facility (“BAF”) with a targeted commissioning for Q4 2022. Companies in the collaboration have supply links to Panasonic-Tesla.
NEXT trades on a market cap of C$296 million and certainly could be the “next” graphite producer.
Syrah Resources Limited (ASX: SYR)
Syrah Resources (“Syrah”) 100% own the world’s largest and lowest cost graphite mine known as the Balama graphite mine, located in Mozambique. It has a 50+ years expected mine life. The past year’s low graphite prices forced the mine to dramatically reduce output but in their September 2021 Quarterly Report Syrah stated: “Balama delivered excellent monthly operational performance for September 2021 with 15kt natural graphite produced at 85% recovery and C1 cash costs (FOB Nacala) of US$430 per tonne…..Strong growth in sales order book with more than 50kt of natural graphite sales orders in the December 2021 quarter, demonstrating robust underlying demand conditions and forward contracting.” So, production is ramping back up again and demand is now running in excess of supply.
Syrah is also working to become a vertically integrated producer of natural graphite Active Anode Material (“AAM”). Syrah has built a pilot AAM facility at Vidalia, Louisiana, USA. The facility has produced initial product samples that are being tested by potential off-takers. The initial stage plan is to ramp up to 10,000 tpa of AAM with discussions ongoing about a larger ramp.
Syrah Resources trades on a market cap of A$640 million. Certainly not a huge market cap for the world’s premier graphite producer. It looks like there are better times ahead for Syrah Resources.
Talga Group Ltd. (ASX: TLG)
Talga Group (“Talga”) 100% own a number of graphite projects located in northern Sweden. Their three advanced projects are Vittangi, Jalkunen and Raitajärvi. Combined they contain JORC resources of 55.3Mt @ 17.5% Cg for 9.7Mt total contained natural graphite. Permitting is underway at their leading Vittangi Project.
Talga has also developed a coated natural graphite anode product (Talnode®-C) and a graphene silicon composite electrode additive (Talnode®-S).
Talga has signed a non-binding LOI with LKAB and Mitsui for a potential JV and development partnership in the mine-to-anode production operation.
Talga Group trades on a market cap of A$472 million. One to watch.
Triton Minerals Limited (ASX: TON)
Triton Minerals (“Triton”) 100% own the Ancuabe Graphite Project in northeast Mozambique. The Ancuabe JORC Ore Reserve is 24.9Mt at 6.2% TGC for 1.544 million tonnes of contained graphite. The December 2017 PFS was based on 60,000 tpa production supporting a mine life of 27 years. The pre-tax NPV10% is US$298 million, and pre-tax IRR is 36.8%. Pre-production CapEx is estimated at US$99.4 million.
In 2019, China’s Jigao International Investment Development Co (a subsidiary of Jinan Hi Tech) invested $19.5 million into Triton Minerals to become a strategic partner. Ancuabe has received final approval for development (mining concession granted and environmental approval), and has ~50% of anticipated Ancuabe production secured by binding off-take agreements. Jigao is assisting with further off-take and Project funding.
Triton plans next to build a commercial Pilot Plant which can be ramped up into production in the near term to produce commercially viable quantities of concentrate to prove the viability of the Project.
Triton Minerals trades on a market cap of A$38 million. Triton did have a problem at their other project called Balama North Project (Nicanda Hill) (lost their lease) which may have hurt sentiment for the stock. Still looks very cheap with strong Chinese support and a low initial startup CapEx.
Other graphite related stocks to watch in 2022
NEO Battery Materials Ltd. (TSXV: NBM | OTCQB: NBMFF). Silicon anode and graphite-silicon anode company. You can read about NEO here.
Magnis Energy Technologies Limited (ASX: MNS) – Graphite development project plus Li-ion battery factories on the way.
Zentek Ltd (TSXV: ZEN) (formerly ZEN Graphene Solutions). A dynamic graphene/ nanotech/ health company. You can read a recent article here that discusses their amazing progress.
Closing remarks
2022 looks like being ‘graphite’s time to shine’ after many tough years. Assuming EV sales continue to grow strongly in 2022 then a graphite shortage looks likely. If this occurs, then it would not be hard to see graphite prices tripling in 2022, just as lithium prices have increased 5x in 2021.
The graphite producers with low costs, ability to rapidly scale production, and ideally offer value-added products should perform best. The graphite juniors that can rapidly progress their projects can also do very well.
Western countries forge green alliance for getting electric vehicle minerals
Originally posted on Mining.com
The founding members of the Sustainable Critical Minerals Alliance. Credit: Jonathan Wilkinson’s official Twitter page
The United States and other western countries on Monday announced an alliance to produce and buy critical minerals from countries with stronger environmental and labor standards, a move that could reduce business with market leader China.
Announced at the COP15 talks on biodiversity in Montreal, the Sustainable Critical Minerals Alliance would support these standards for elements like lithium, cobalt and nickel, Canada’s Natural Resources Minister Jonathan Wilkinson said.
“Unless China and Russia are willing to put in place … measures required to be able to legitimately say that they are supporting these kinds of standards then it would essentially mean … we will be buying alternatives as we can,” Wilkinson said in an interview.
Wilkinson acknowledged that the voluntary alliance of the United States, Canada, Australia, France, Germany, Japan and the United Kingdom would not shun China which dominates the market for the minerals used in EV batteries.
“Obviously right now there are some critical minerals that are processed in large measure in China so this will be something that will need to happen over time,” he said.
Western countries have been trying to wean themselves from dependence on authoritarian regimes for strategically important materials. Canada last week unveiled a strategy to ramp up production and processing of critical minerals. In June, the United States and allies set up a partnership aimed at securing supplies.
China said it has taken steps to curb pollution in its mining sector, but has faced criticism.
Mining, along with other sectors are under scrutiny at the Montreal talks due to their impact on nature.
“China is actually free to up its game with respect to environmental standards and with respect to labor standards and eventually join the alliance,” Wilkinson said. “But it would have to make those kinds of changes.”
A strategist from environmental group Greenpeace welcomed the alliance’s support for higher environmental, indigenous rights and labor standards but questioned how it would be enforced.
“Will there be teeth to that? For the moment it’s more like a memorandum,” said Keith Stewart, senior energy strategist, Greenpeace Canada.
(By Allison Lampert; Editing by David Gregorio)
Canada’s mining minister wants minerals projects built within a decade
Originally posted on Mining.com
Canada’s Minister of Natural Resources Jonathan Wilkinson. (Image courtesy of Province of British Columbia.)
Canada’s mining minister wants critical minerals projects built in less than a decade — spurred on by government efforts to cut red tape.
“We need to get to the point where we can get these mines from concept to production certainly within a decade, and ideally less than that,” Natural Resources Minister Jonathan Wilkinson said in a Monday phone interview.
Wilkinson’s comments come days after his ministry published a critical minerals strategy that pledged to review Canada’s approval process for developing mines. Government estimates show it can take up to 25 years for a mining project to become operational. Wilkinson said he expects policy recommendations on streamlining processes within the next 12 months.
The time it takes to build a mine has been a source of concern for mining companies worldwide, given that lengthy approval processes pose investment risks and heightened costs, and is top of mind for many mining CEOs. The head of Vancouver-based Teck Resources Ltd., for instance, said last week that the Canadian government could help the industry with an approval process that ensures projects get done in a timely fashion.
“If we are going to bring supply online at the pace that the world needs to electrify, we need to shorten those timelines,” Chief Executive Officer Jonathan Price said in a Thursday interview. “Getting the approvals pathway right is very important, but we have to look for opportunities to accelerate so we can bring new production to market more quickly.”
High-Performance Amorphous Carbon Coated Lithium-Nickel-Manganese-Cobalt-Oxide (NMC622) Cathode Material with Improved Capacity Retention for Lithium-Ion Batteries
Originally posted on wevolver.com
This article is a part of our University Technology Exposure Program. The program aims to recognize and reward innovation from engineering students and researchers across the globe.
Coating conducting polymers onto active cathode materials has been proven to mitigate issues at high current densities stemming from the limited conducting abilities of the metal-oxides. In the present study, a carbon coating was applied onto nickel-rich NMC622 via polymerisation of furfuryl alcohol, followed by calcination, for the first time. The formation of a uniform amorphous carbon layer was observed with scanning- and transmission-electron microscopy (SEM and TEM) and X-ray photoelectron spectroscopy (XPS). The stability of the coated active material was confirmed and the electrochemical behaviour as well as the cycling stability was evaluated. The impact of the heat treatment on the electrochemical performance was studied systematically and was shown to improve cycling and high current performance alike. In-depth investigations of polymer coated samples show that the improved performance can be correlated with the calcination temperatures. In particular, a heat treatment at 400 °C leads to enhanced reversibility and capacity retention even after 400 cycles. At 10C, the discharge capacity for carbon coated NMC increases by nearly 50% compared to uncoated samples. This study clearly shows for the first time the synergetic effects of a furfuryl polymer coating and subsequent calcination leading to improved electrochemical performance of nickel-rich NMC622.
Introduction
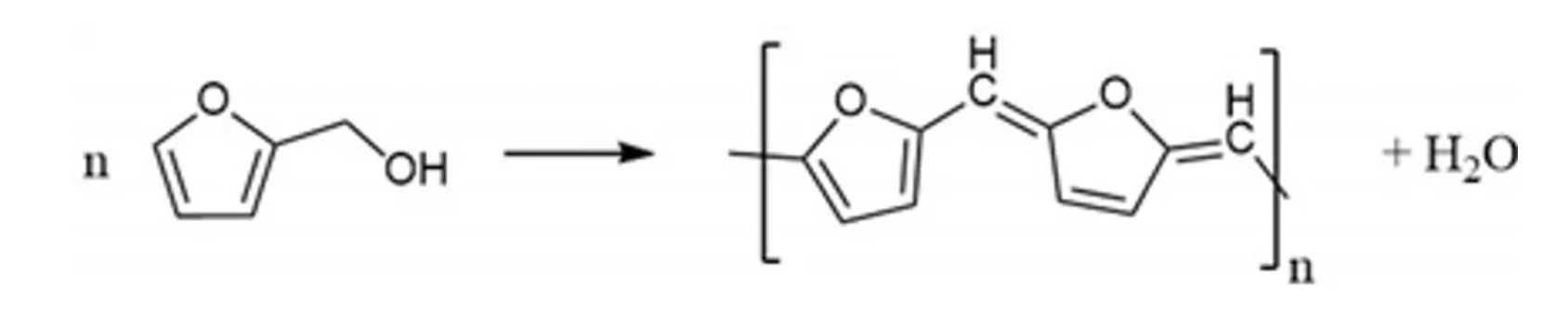
Ni-rich NMC, LiNixMnyCozO2 (where x ≥ 0.5, x + y + z = 1) has attracted great attention in the battery community due to a combination of high reversible capacity (180–250 mAh g−1) and high operating voltage (~3.8 V vs. Li+/Li) that stems from two-dimensional lithium-ion diffusion and good lithium-ion conductivity [1]. However, in spite of these advantages, the material is known to possess problems such as surface side reactions and chemical instability at the highly de-lithiated stages (>4.3 V vs. Li+/Li) [2,3,4,5]. To overcome these issues and to achieve the long-term performance of Ni-rich NMC materials, surface stabilisation of Ni-rich materials is considered an efficient strategy [3,4,5,6,7,8,9]. Such surface coating of materials/layers should offer decreased surface impedance, unchanged Li+ diffusivity, and chemical stability vs. the electrolyte throughout the applied voltage window. Metal oxides, including Al2O3 [10,11,12], ZrO2 [13,14], MgO [15], Li2O–2B2O3 [16], TiO2 [17], SiOx [18], ZnO [19], SnO2 [20], Y2O3 [21], LiNbO3 [22], and LiAlPO3.93F1.07 [23] have been employed as coatings which improved the electrochemical performance and stabilised Ni-rich NMC materials during prolonged cycling. The delay in structural degradation stems from an effective protective layer that prevents the material from HF-based surface side reactions and lowers the charge transfer resistance of Li+ and transition metals dissolution. However, their inherently low electronic conductivity results in a poor electrochemical performance at high current rates, which challenges their ability in high power applications [24,25,26,27]. To solve this problem, a carbon coating on the surface of metal-oxide particles is one of the strategies demonstrated in the literature. Many studies demonstrated that a nanometre layer of carbon increased electronic conductivity of the cathode material and reduced side reactions with the electrolytes. The layer creates a physical barrier between the electrolyte and the metal-oxide-based cathodes [24]. This has been widely applied for low conducting materials such as LiFePO4 [28,29] and Li2FeSiO4 [30,31]. Hence, thin layers of carbons are a proven strategy to increase the electrochemical performance at high current rates. However, achieving homogeneous coatings on micrometre-sized commercial NMC cathode materials remains challenging. In this regard, amorphous carbon-based materials from polymerisation offer homogeneous coverage and high electronic conductivity. Their good chemical and electrochemical stability have been reported as promising alternatives to metal-oxide-based coatings [32]. Moreover, low material cost and processes makes them potentially competitive with metal-oxide coatings. Compared to nanosized materials, large particles of a mean diameter of ~10 µm were found difficult to coat and only a thin layer on the material can be obtained through a general solution-based coating approach. Furthermore, it is well known that in inert atmosphere carbon acts as a reducing agent and can remove oxygen from the NMC structure, leading to the formation of unwanted surface species and material degradation [33]. Therefore, in this work we present a method that reduces the risk of oxidizing the carbon layer by a process that involves polymer curing on NMC particles, and the formation of carbon at elevated temperatures is carried out in air atmosphere. Here, we have performed an amorphous polymer coating of poly-furfuryl on NMC622 through the polycondensation of furfuryl alcohol (Equation (1)). In detail, the process contains the steps of monomerpolymerisation at 80 °C, followed by curing of the polymerised products at 120 °C to form a cross-linked polymer structure. A final calcination step creates a uniform amorphous carbon layer.
Although there are approaches to obtain carbon coated Ni-rich NMC through solid state methods, these examples restrict control over thickness [24,34]. On the other hand, polymerisation of organic monomers on top of metal-oxide particles followed by calcination allows for the formation of amorphous carbon with a defined thickness. Hence, in the present study we performed carbon coatings on NMC622 micro-sized particles through the polymerisation of furfuryl alcohol (FA) followed by a calcination step. A systematic study of carbon coating thickness and heat treatment on NMC622, comparing coated with uncoated micro particles that improve the electrochemical performance, is presented in this work. Furfuryl alcohol is used as fire retardant and for its thermosetting properties after polymerisation [35] as well as its ability to form carbon layers with good mechanical properties. It is also known to be chemical inert towards corrosive species, such as HF [36]. Here, we show that a uniform coating was achieved by polycondensation of furfuryl alcohol followed by calcination of the cross-linked polymer structure in air. Further, a detailed study on the effect of variation of calcination temperature of the poly-furfuryl coated NMC622 on the thickness of carbon coating was performed and revealed optimum conditions at 400 °C. A nearly 15 nm thick layer of amorphous carbon on NMC622 particles can be derived from the calcination of poly-furfuryl, which results in increased capacity retention and higher discharge capacity of up to 10 C during discharge.
Results
Polymer Formation
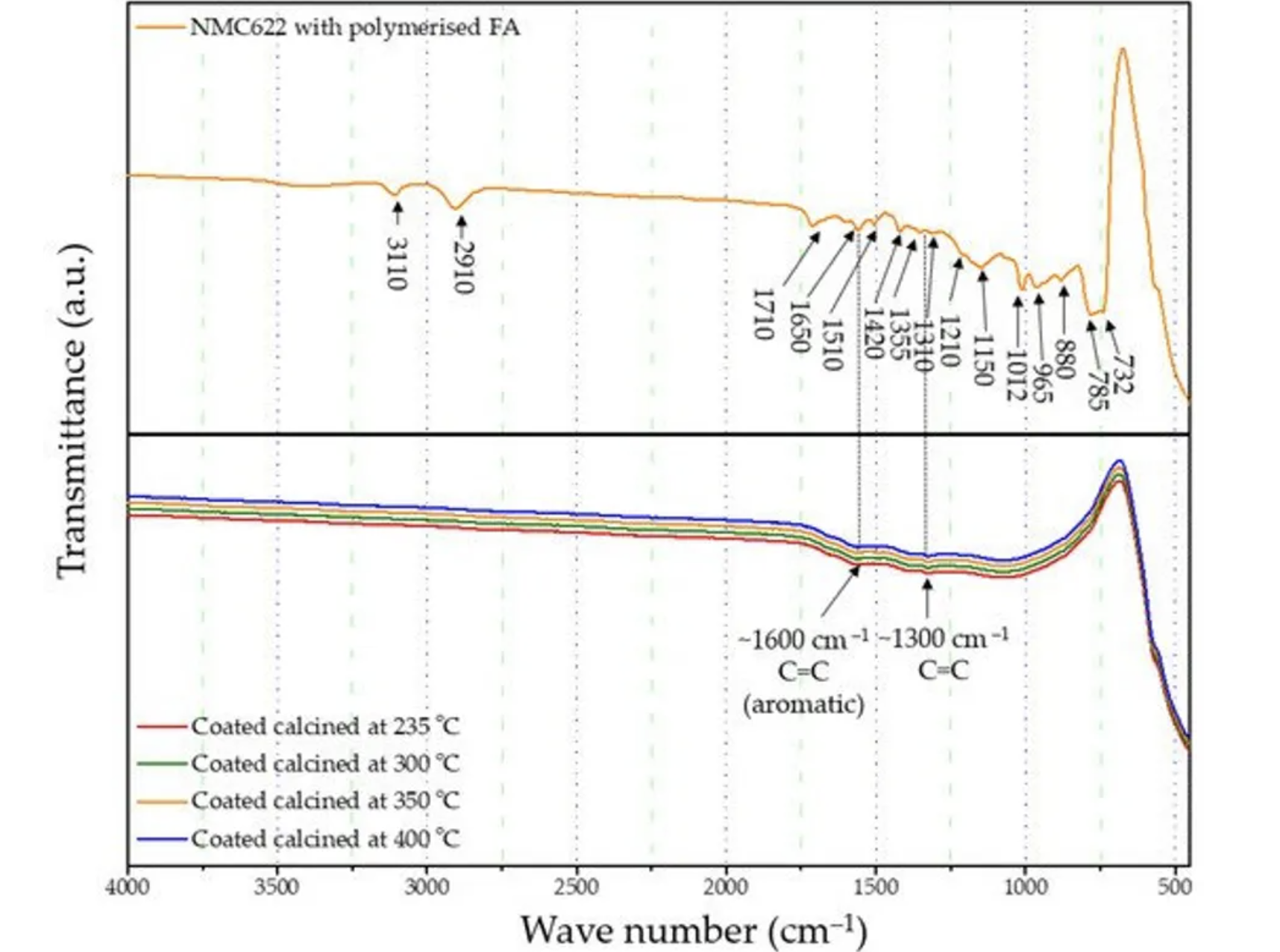
The formation and curing of the polymer were confirmed by the Fourier-transform infrared spectroscopy (FTIR) analysis shown in Figure 1. The broad OH peak in the region 3600–3000 cm−1 is strongly reduced, indicating that polycondensation of the monomer took place. Moreover, increased vibration bands in the spectral range of 1710 and 1520 cm−1 indicate the presence of C=O and C=C stretching, respectively, which implies the successful formation of cross-linkage via the Diels–Alder reaction followed by ring opening. These functionalities, on the other hand, cannot be detected in the furfuryl alcohol monomer [37].
Figure 1. FTIR analysis of the furfuryl alcohol monomer and cured polymerised coated NMC622.
Polycondensation reaction
(1)
Thermal Decomposition of the FA Polymer
The thermal decomposition of the cured polymer coating on top of NMC622 was investigated by thermogravimetric analysis/ differential thermal analysis (TGA/DTA) and mass spectrometry. NMC coated with cured FA-polymer was loaded in a corundum crucible and subjected to thermal analysis. The sample was heated from room temperature up to 500 °C with a heating rate of 5 °C/min. Weight loss and differential temperature was recorded, and the evolved gases were analysed. A weight loss of ~3% between room temperature and 410 °C can be divided into four regions (30–100 °C, 100–310 °C, 310–410 °C, 410–480 °C) as shown in Figure 2a. In region 1a, weight loss of ∼0.42 wt.% in the temperature range of 30–100 °C is observed and can be attributed to the desorption of residual water stemming from the polycondensation reaction and humidity. Such low quantities are below the detection limit of the mass spectrometer and are therefore not visible. In region 2 and 3, the weight loss of ~2.6 wt.% between ~100 and 410 °C can mainly be assigned to the decomposition of the polymer coating and formation of a carbon species as the function of the temperature; above 410 °C, weight loss becomes insignificant. Therefore, 400 °C was chosen as the optimum temperature with the objective to obtain a thin-layer carbon coating on top of NMC622 particles. The DTA curve shows two exothermic peaks during the heating process; to understand the mechanism behind the decomposition, TGA/DTA coupled mass spectrometry was carried out with the mass to charge ratios m/z of 18 and 44 datapoints corresponding to H2O and CO2, respectively (Figure 2). Between 30 and 100 °C, region 1 shows an endothermic peak which is characteristic for the evaporation of water. In contrast to region 1, region 2 (100–310 °C) is characterised by an exothermic peak together with the evolution of water and CO2. In the literature it is well described that the rupture of furan rings leads to the formation of amorphous carbon species and subsequent evolution of CO2 [38], which indicates the decomposition of the organic polymer structure. The appearance of H2O at an onset temperature of 250 °C can be explained as the remaining monomer undergoing a polycondensation reaction. In region 3, a second exothermic process starts at 330 °C which finds its maximum at 390 °C. The appearance of only CO2 correlates to further decomposition of the carbon-based structure.
Figure 2. (a) TGA/DTA of the polymer coated NMC622 carried out in synthetic air atmosphere at 5 °C/min and 50 mL/min flow. (b) Mass spectroscopy of water (m/z 18) and carbon dioxide (m/z 44) of the respective TGA/DTA.
Structural Stability of Coated NMC622
It is well known that carbon is a reducing agent and thus can reduce the transitional metal in the NMC structure, leading to oxygen release in the structure and the formation of CO/CO2 gases [8,33]. Hence, layered NMC has the tendency to transform to spinels and rock-salt structures upon heating in the presence of carbon [33]. Therefore, considerable efforts have been devoted during the calcination process to obtain carbon from polymeric organic materials in an air atmosphere while maintaining the layered NMC structure. In order to verify the structural stability of the NMC622 upon systematic heating, a study on the calcination of the poly-furfuryl coated particles was carried out at 235 °C, 300 °C, 350 °C, and 400 °C as shown in Figure 3. To investigate changes in the polymer structure where initiation of decomposition was expected, 235 °C was chosen as the lowest temperature and the upper limit was set at 400 °C since the mass loss at higher temperatures appeared insignificant. Powder X-ray diffraction at elevated temperatures revealed that all materials remained single phase NMC622 in the R-3m space group [1]. Clear peak splitting at ~38° and ~65° 2θ corresponds to (006)/(102) and (108)/(110) hkl planes and proves the high crystallinity of the materials after coating and heating. Lattice parameters obtained from Rietveld refinement are given in Table 1. It has been reported that the intensity ratio of (003)/(104) indicates cation mixing in the lattice of NMC622 material, and that values of (003)/(104) > 1.2 indicate no obvious cation mixing in the structure [1,39,40], as was found for the coated materials described here (Table 1). Also, the c/a ratio of >4.91 strongly indicates hexagonal ordering of the coated materials and the exclusion of cation-mixing [39]; this further proves the thermal stability and remaining crystal integrity of NMC622 throughout the polymerisation process and subsequent heat treatments.
Figure 3. XRD patterns of (a) uncoated pristine NMC622 and coated calcined NMC622 at (b) 235 °C, (c) 300 °C, (d) 350 °C, and (e) 400 °C for 120 min duration.
Table 1. The lattice parameters of the coated/calcined and uncoated NMC622 samples.
Structure of the Polymer Coating
FTIR-ATR analysis of the uncoated pristine NMC showed weak bands between 400 and 520 cm−1 corresponding to the O–M–O asymmetric bending modes, and above 520 cm−1 asymmetric stretching modes of the MO6 (where, M=Ni, Co, Mn) octahedra (Figure 1) [41,42]. The main characteristic bands for the cured polymer coated on NMC622 were seen at 3110 cm−1 (–CH in aromatic rings), 2910 cm−1 (aliphatic –CH stretch), 1710 cm−1 (C=O stretch), 1650 cm−1 (C=C stretch), 1510 cm−1 (ring vibrations), 1420 cm−1 (asymmetric –CH2 bending), 1355 cm−1 (–CH ring stretch), 1210 cm−1, 1150 cm−1 (C–C furan stretch), 1012 cm−1 (=C–O–C= furan ring stretch), 880 cm−1 (=C–H= furan ring stretch), 785 cm−1 (twisting of –CH ring structure), and 732 cm−1 (–CH ring out of plane stretch) [43]. However, all vibrations in the range of 1675–1015 cm−1 disappear after the calcination process and the band corresponding to the carbonyl species at 1715 cm−1 decreases in intensity. At the same time, two main broader bands increase at ~1600 and ~1300 cm−1 (Figure 4), which are described in the literature as the formation of amorphous carbon and carbon nanotubes [44,45]. Only weak intensity bands can be observed in Figure 4, which were caused by the low quantity of the amorphous carbon due to the thin nature of the coating remaining on the samples after the heat treatment. These absorption bands show proof of the conversion from the poly-furfuryl alcohol polymer into an amorphous carbon-like structure with an aromatic character [46]. Based on these findings, the successful transformation of the poly-furfuryl into the carbon structure can be confirmed.
Figure 4. FTIR spectrum of uncalcined polymer coated NMC622 and all calcined coated NMC622 samples.
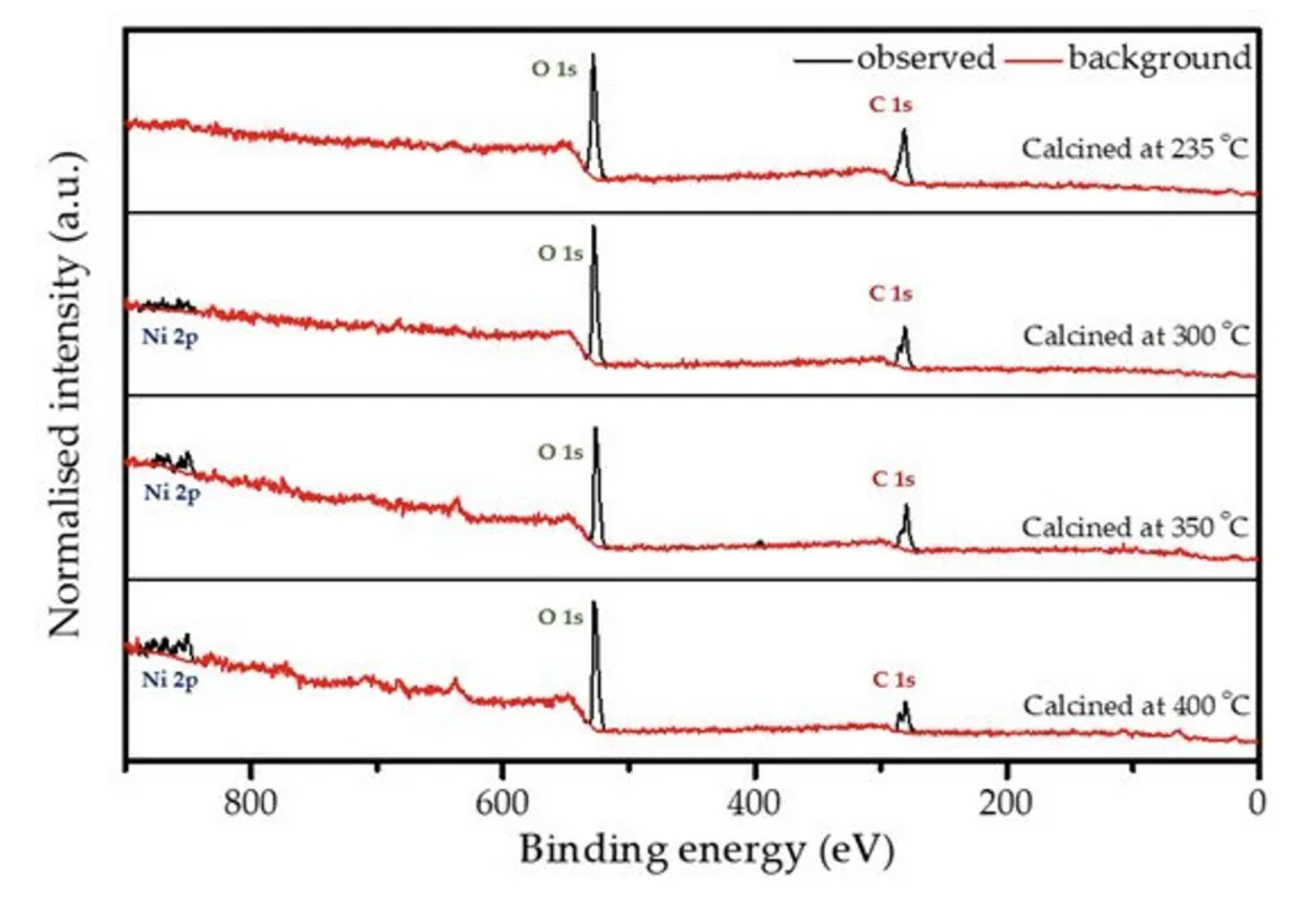
X-ray photoelectron spectroscopy (XPS) analysis was performed on all calcined samples to confirm the presence of carbon species and to determine the variation in elemental composition of the surface layer throughout the calcination process. Figure 5 shows the full XPS survey spectra of all the coated and calcined powder samples, which contain the expected elements C and O on all samples, as well as Ni. However, the sample heated to 235 °C does not show a significant peak for nickel (see also Figure S2b), which can be explained by changes in coating thickness with increasing calcination temperature together with the limited depth sensitivity of the instrument of about 7–10 nm. When increasing the calcination temperature, the carbon content decreases, while O and Ni increase as shown in Figure S2b and Table 2, which points towards the removal of the carbon layer. An anomaly is observed at 350 °C due to the partial surface charging during the measurements, even though it was minimised, but not fully suppressed by using a low energy electron flood gun for charge compensation. The C1s spectrum shown in Figure S2a consists of two peaks. The high intensity peak at 285.0 eV is attributed to the amorphous carbon [36] present in the sample, whereas a low intensity peak at 289.2 eV originates from carboxylic groups [47]. The resulting amorphous carbon derived from poly-furfuryl alcohol is known for its excellent combination of mechanical and optical properties, i.e., extreme hardness and inertness against acid environment (HF included) [36,48]. Results from FTIR analysis and XPS measurement confirms a thin layer of carbon and the amorphous nature of the structure obtained from the calcination process of poly-furfuryl.
Figure 5. XPS survey spectra for the coated powder samples that are calcined at different temperatures.
Table 2. Elemental quantification from XPS (estimated error =10% for values >10 at% and 15–20% for <10 at%).
Morphology of the Polymer Coating
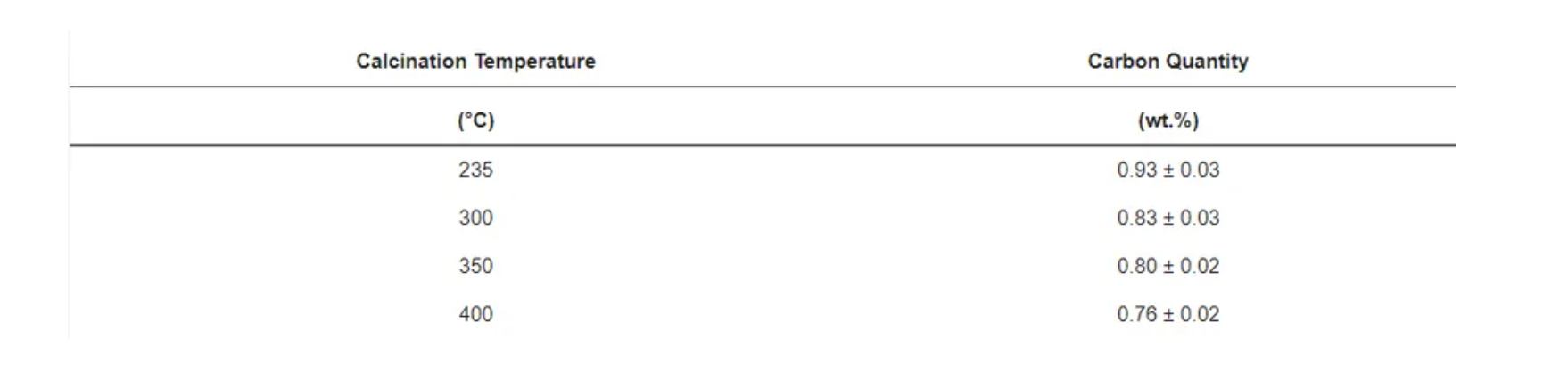
The morphology and microstructure of uncoated pristine, coated, and calcined powders were investigated by scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analysis. SEM of the uncoated pristine sample (Figure 6a) shows 200–500 nm primary NMC622 particles with rough surfaces, and secondary spherical particles of 5–15 µm size. Figure 6b shows a complete coverage of the NMC particles with the polymer. Furthermore, calcination of the polymer structure (Figure 6c–f) led to a homogeneous carbon coated NMC622 material, as can also be observed via elemental mapping with energy dispersive X-ray spectroscopy (EDX). The results of the detailed EDX analysis (see Figure S3) show the loss of carbon for the coated sample as the calcination temperature increases. CHN analysis was carried out to determine the amount of carbon derived from poly-furfuryl calcination (Table 3); it shows that an increase in temperature results in the decrease of carbon content. The findings are in line with TGA data, which show a mass loss with increasing temperature.
Figure 6. SEM images of (a) Uncoated NMC622 (b) NMC622 after 120 °C curing, (c) At 235 °C calcinated sample, (d) At 300 °C calcinated sample, (e) At 350 °C calcinated sample, (f) At 400 °C calcinated sample for 120 min. duration.
Table 3. Carbon quantification after calcination derived from CHN.
TEM was carried out to examine the thickness of the coating after each temperature calcination and confirms a uniform layer of carbon around the surface of the NMC secondary particles (Figure 7). It clearly shows the effect of temperature on the polymer layer thickness. We observed a~100 nm thickness for samples calcined at 235 °C, ~50 nm at 300 °C, ~30 nm at 350 °C, and ~15–20 nm at 400 °C. Furthermore, a uniform carbon layer was observed by surface mapping; the results are presented in Figure 7f–h. The images provide a clear picture of a homogeneous carbon coating on top of the NMC particles. However, the diffused ring in the coated area indicates the amorphous nature of the carbon (Figure 7e) [49]. These findings are in line with the XPS, EDX, and CHN analyses, which depict a strong dependency of coating thickness to the calcination temperature resulting in uniform carbon layers.
Figure 7. TEM images of polymer coated samples calcined at (a) 235 °C, (b) 300 °C, (c) 350 °C, and (d) 400 °C. (e) SAED pattern of the carbon derived from 235 °C calcination from the area highlighted in (f). (f) Mapped image of 400 °C, (g) mapping of all elements (Ni, Mn, Co, C), and (h) elemental mapping of carbon only.
Electrochemical Performance
Cyclic Voltammetry
To investigate the influence of the coating on electrochemical performance, cyclic voltammetry (CV), charge–discharge, rate-capability tests, and impedance studies were carried out. Figure 8a shows the first cycle of CV comparing uncoated pristine and coated/calcined samples. For the uncoated pristine material, an oxidation peak at ~3.85 V vs. Li/Li+ and a reduction peak at ~3.70 V vs. Li/Li+ during the first cycle can be observed, corresponding to oxidation and reduction of the Ni2+/4+ redox couple [1]. Cyclic voltammetry revealed that coating thickness and temperature had no impact on either the uncoated or the coated materials upon cathodic scan, showing a stable reduction peak (Figure 8a). However, in the first cycle the voltage difference between the anodic and cathodic peaks was higher for the coated samples compared to the uncoated ones (~0.126 V), and as the temperature of calcination increased to 235 °C, 300 °C, 350 °C, and 400 °C, the voltage difference decreased to 0.273 V, 0.235 V, 0.194 V, and 0.156 V, respectively. The increase in the voltage difference can be mainly attributed to a shift to higher potential for the first anodic peak. This can be explained by a hindered Li+ diffusion in the first anodic scan, which was exacerbated with the increase in thickness of the coated layer on top of the particles. However, from the second scan onwards the offset of the anodic peak shifted to lower potentials, since all the coated calcined samples were forming stable cathode electrolyte interfaces (CEIs) [2]. During subsequent cycles, Ni2+/4+ redox couples remained unchanged for the carbon coated samples.
CCCV Charge and CC Discharge
In CCCV experiments, typical charge–discharge profiles for NMC type materials were observed for pristine and calcined NMC622 cathode materials in the 1st, 200th, and 400th cycle, which are shown in Figure 8b, Figure 8c, and Figure 8d, respectively; testing was conducted at 2 C (where 1 C = 160 mAh g−1) in the potential window of 3.0–4.3 V vs. Li/Li+. All cells were subjected to formation cycles at 0.1 C for the first five cycles, to form a stable CEI layer at the interface to adjust for high discharge loads in the successive cycles. All tests for NMC show typical progressive and retrogressive charge–discharge curves (Figure 8b). When comparing the formation cycles of freshly prepared cells, a similar behaviour in relation to CV measurements can be observed. The overpotential increased (higher charge plateaus) during charging for calcined in comparison to uncoated materials and most likely can be related to a reduced Li+ ion diffusion. This phenomenon can be clearly related to the thickness of the coating (see Figure S4). Also, CCCV experiments gave similar discharge curves compared to CV for all samples. During the first cycle, the charge plateau for uncoated samples started at ~3.70 V, at ~3.74 V for the coated ones, and it continued to increase to ~3.77 V as the thickness of the coating increased. A potential increase at the very beginning of the charge curve can be observed, which is attributed to the change of the current rate (0.1 C to 0.5 C) after formation, the thickness of the coating layer, and surface impurities [8]. However, the discharging profiles of pristine and calcined materials are similar without noticeable difference in the ohmic drop (IR drop). After 200 cycles, an increase in the IR drop can be seen with a similar charge–discharge behaviour, which is mainly due to the rise of internal resistance, as shown in Figure 8c. This was caused by the accumulated products from side reactions over 200 cycles and resulted in the drop of potential at the beginning of discharge. However, the IR drop or ohmic polarisation was less in the case of calcined compared to uncoated materials and showed its lowest values for the thinnest coatings. Similarly, the concentration polarisation was lower for calcined compared to the uncoated samples, which is attributed to the mass transfer within the cell during charge–discharge. This further confirms an improved charge–discharge performance for carbon coated samples. It can also be assigned to the better percolation network, which improves the electronic conductivity of the particle and leads to fewer side reactions stemming from the metal-oxide surface. Likewise, after the 400th cycle, much higher polarisation losses can be observed in the uncoated sample compared to the calcined material (seen in Figure 8d). The increase in polarisation was mainly caused by the increase in internal resistance, which may have originated from surface side reactions. Figure 8e shows the cycling performance of the uncoated and cells with calcined NMC materials over 400 cycles, performed at a 2 C discharge rate. The discharge capacity at the first cycle after formation for NMC powders calcined at 235 °C, 300 °C, 350 °C, and 400 °C delivered values ranging from 153.59 mAh g−1 to 156.98 mAh g−1. The same order of capacity decrease can be observed after completing 400 cycles with discharge capacities ranging from 125.80 mAh g−1 to 140.36 mAh g−1 as shown in Table 4. As a result, higher capacity retention can be observed in the coated materials, which increased with thinner coatings (85.29%, 85.56%, 86.93%, and 89.42%). In contrast, the uncoated NMC622 shows only 81.38% from the first cycle as shown in Figure 8f. Hence, coated calcined samples give better performance and higher capacity retention upon long-term cycling and it is believed this performance improvement is directly related to the amorphous carbon surface coating. A comparison of previously reported studies related to the long-term cycling stability of coated materials can be found in Table S2, highlighting the improved capacity retention that can be achieved with the materials reported in this work.
Table 4. Specific discharge capacities (in mAh g−1) at 2 C of uncoated pristine compared to calcined samples after the 1st and 400th discharge cycle (standard deviation = ±2 mAh g−1).
Cycling Experiments of Heat Treated Uncoated Pristine NMC622
To confirm the effect of the calcination on the performance improvement of the NMC material, samples treated at elevated temperatures, but without carbon coating, were tested under the same conditions and compared with the coated samples. Rietveld refinement analysis was carried out and is presented in Figure S5 and Table S1. The clear peak splitting at ~38° and ~65° 2θ corresponds to (006)/(102) and (108)/(110) hkl planes, which indicates the high crystallinity of the materials after heating. Lattice parameter values of (003)/(104) >1.2 indicate no obvious cation mixing in the structure [1,39,40]. Their cycling performance was tested under the same conditions as the coated samples, depicted in Figure S6. Specimens calcined at different temperatures, namely, 235 °C, 300 °C, 350 °C, and 400 °C, delivered discharge capacities after the first cycle ranging from 155.18 mAh g−1 to 156.46 mAh g−1. For cells cycled 400 times, discharge capacities in the range of 128.89 mAh g−1 to 134.27 mAh g−1 were observed, which gives capacity retentions of 83.06%, 84.28%, 84.37%, and 85.81%, respectively as shown in Table 5. These findings are in agreement with published data where the improvement in capacity retention originates from the removal of the surface impurities (LiOH, Li2CO3) during the heat treatment, and from the enhancement of crystallinity due to the heating step [3,7,8]. Hence, the heat treatment of NMC particles results in improved performance. However, the amorphous carbon coating presented in this paper further improves performance during the long-term cycling and has a high C-rate testing.
Table 5. Specific discharge capacities (in mAh g−1) at 2 C of uncoated pristine samples after the 1st and the 400th discharge cycle (standard deviation = ± 2 mAh g−1).
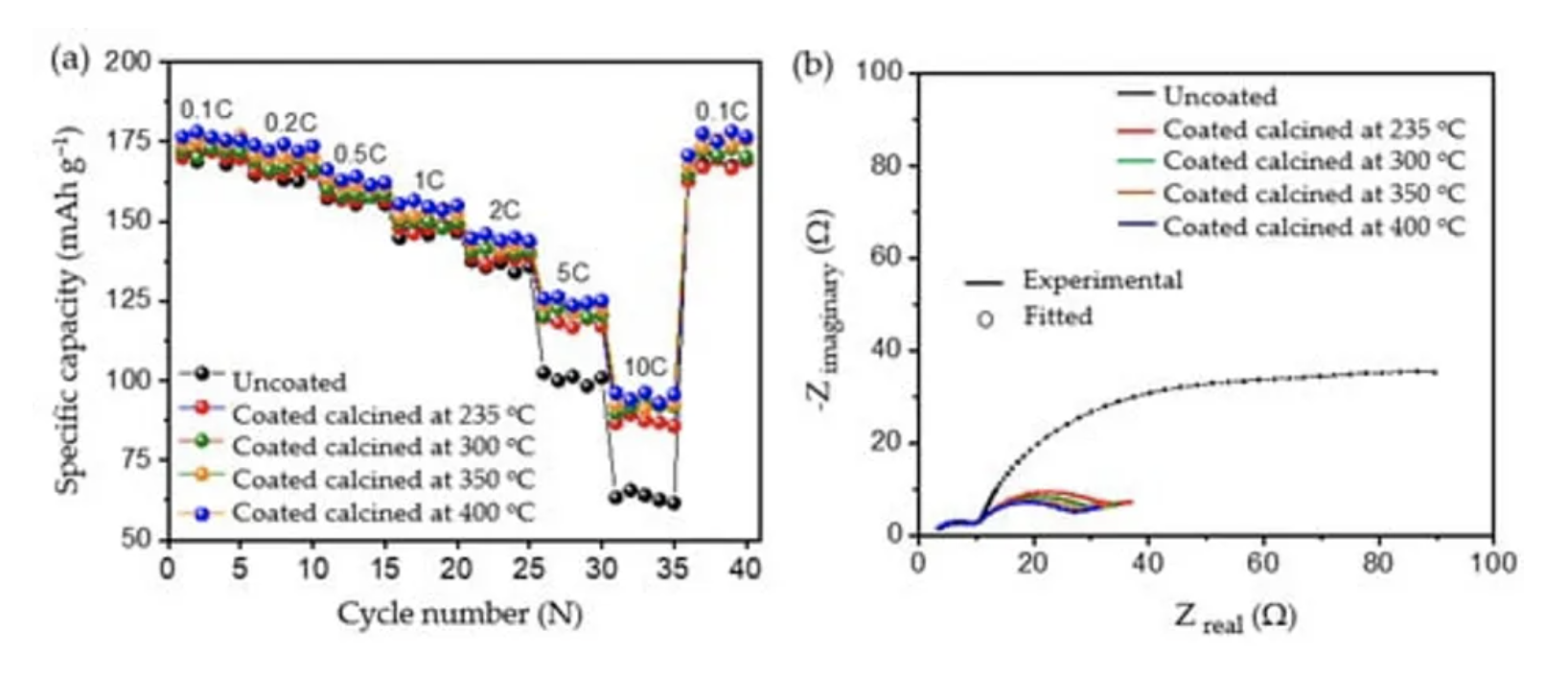
Rate Capacity and Electrochemical Impedance Spectroscopy Test
To evaluate the robustness of the carbon coatings at different loads of current, rate capability testing was carried out as shown in Figure 9a. At lower current rates such as 0.1 C, 0.2 C, 0.5 C, 1 C, and 2 C, both the uncoated and coated materials delivered similar discharge capacities, without significant differences in discharge performance. However, when higher loads of current such as 5 C and 10 C were applied, all the coated calcined samples delivered significantly increased discharge capacities compared to the uncoated pristine material; see Table 6. The improvement in the performance of the coated calcined material is directly proportional to the thickness of the coating layer; e.g., by reducing the thickness of the coating from ~100 nm (coated calcined at 235 °C) to ~15 nm (coated calcined at 400 °C), the discharge capacity at 10 C increased from 87.23 mAh g−1 to 94.97 mAh g−1, which relates to a nearly 9% capacity improvement. Also, when compared with the uncoated sample, which delivered only 63.41 mAh g−1 at 10 C, the calcined material at 400 °C showed a nearly 50% improvement in the discharge capacity. Comparing high current tests of NMC622 coated with ~15 nm amorphous carbon from this work with state-of-the-art coated materials shows excellent performance for high currents of 10 C at discharge (see Table S3).
Figure 9. Rate capability tests for (a) uncoated and different coated materials and (b) electrochemical impedance spectroscopy for the uncoated and coated electrodes.
Table 6. Specific discharge capacities (in mAh g−1) of uncoated and calcined samples (standard deviation = ±2 mAh g−1).
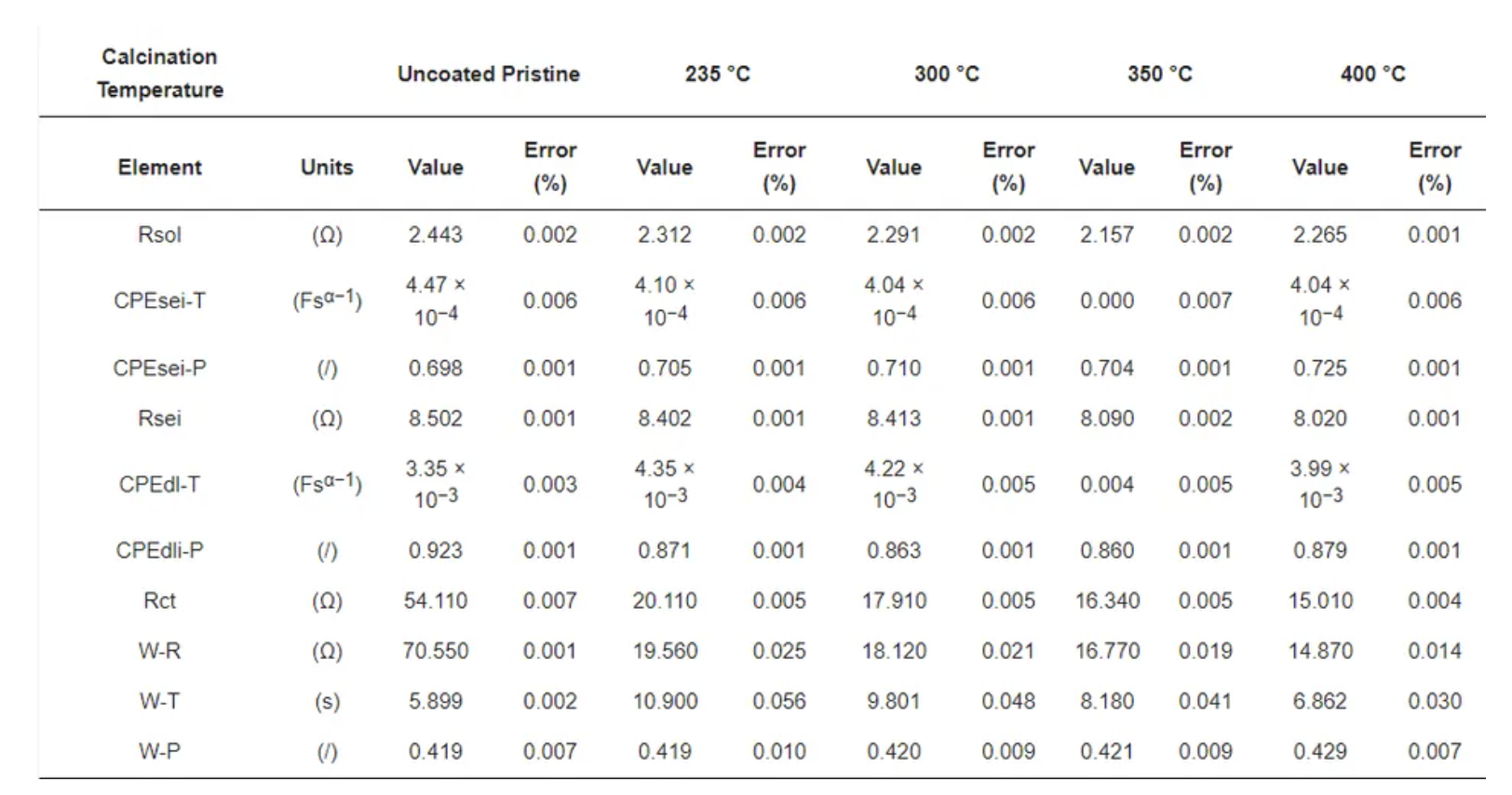
To determine the reason for the presented performance enhancement, electrochemical impedance spectroscopy studies for uncoated and all calcined samples were performed after the formation cycle. Therefore, cells were charged to a nominal voltage (3.8 V) before acquiring impedance data. The results are shown in Figure 9b. From the perspective of the qualitative analysis, the coated calcined samples showed lower charge transfer resistance (Rct) compared to the uncoated samples. That is, the charge transfer resistance of the coated materials decreased with the thickness of the coating, indicating the formation of a thinner and more stable CEI layer on top of the material. Furthermore, the charge transfer resistance for Li+ was reduced by providing optimum encapsulation with amorphous carbon as a layer on top of the particles and as a result improved ionic conductivity. The lower charge transfer resistance of samples calcined at 400 °C may be one of the reasons for the higher rate performance at 10C due to the improved percolation pathways for Li+ movement and enhanced electric conductivity. However, to quantify the improvement, the detailed analysis of the obtained spectra was carried out using the electrical equivalent circuit shown in Figure S7, where Rsol represents the electrolyte and cell resistance The parallel CPEsei and Rsei represent the capacitive nature and resistance of the passivation layer on the electrode–electrolyte interface, respectively (i.e., the small arc at high frequencies region). The next parallel constant phase element (CPEdl) and Rct represent the charge-transfer process (i.e., the dominating arc in the Nyquist plots). The generalised finite space Warburg (GFW) is used to fit the diffusion component of the plot [50]. Since the lithium ion intercalates into the cathode during the discharge process through the SEI layer followed by diffusion in the bulk, the GFW element is added in parallel CPEdl and series to Rct [51]. The constant phase element (CPE) is used instead of the capacitor, due to its non-ideal capacitor nature attributed to the electrode surface inhomogeneity or dispersed charge-transfer reactions [51]. The exponential parameter “P” is used to describe its deviation from the ideal capacitor.
Table 7 shows the obtained best fit values of the uncoated and coated samples, where the low error in the determination of each component shows a good quality of fit and high reliability of the performed fit. The Rsol values do not deviate much during each measurement, indicating high reproducibility in cell assembly and measurements. The lower value of Rsei was found in different samples due to the thin electrodes (low active material loading per area). The variation of Rct in different coating thicknesses was found to be more dominating. The deviation of Rct was found to be from 20.11 Ω to 15.01 Ω with the thickness of the carbon coating ranging from ~100 nm to ~15 nm, respectively. However, the value in the uncoated sample, found to be 54.11 Ω, is much higher, which underlines the positive impact of the amorphous carbon coating on cell performance. The smaller Rct values for the coated samples compared to the uncoated materials relates to the surface deintercalation–intercalation of lithium ions in the cathode during the charge–discharge process. Accordingly, upon comparing the coated and uncoated electrodes, owing to the lower value of charge-transfer resistance in the amorphous carbon coated samples, a better Li+ intercalation–deintercalation was observed. Further, a strong dependency on the thickness of the coating layer to charge-transfer resistance can be seen, where Rct values decrease as the thickness of the coating reduces.
Table 7. Best fit values of uncoated and calcined samples.
Post-Mortem Analysis
Post-mortem analysis was conducted after 400 charge–discharge cycles to reveal the underlying processes that cause improved performance for coated materials compared to untreated NMC. Figure 10 shows the XRD and SEM analyses carried out on the uncoated and NMC calcined at 400 °C. The cells were disassembled inside a glove box and washed gently with dimethyl carbonate (DMC) to remove any residual LiPF6 salt. The XRD of the cycled electrodes shows clear peak splitting of (006)/(102) and (108)/(110) hkl planes for the coated materials, while uncoated pristine NMC622 shows low intense peaks as highlighted in Figure 10; the arrival of new shoulder peaks and the broadening of the (110) peak indicate the partial loss of hexagonal order in the crystal lattice. When comparing electrodes after 400 cycles, specimens calcined at 400 °C show higher crystallinity in comparison with untreated pristine NMC622 (Figure 10a). SEM analysis reveals that heat treatment and galvanostatic cycling has no impact on primary and secondary particle morphology (Figure 10b,c).
Figure 10. (a) XRD of electrodes of uncoated pristine (top) and calcined samples, heat treated at 400 °C. (b) SEM of uncoated samples and (c) SEM of the calcined samples. Electrodes were collected after the completion of 400 cycles.
Materials and Methods
Material Preparation
Commercially available NMC622 (BASF, HED™) with ~10 µm size, was used as the parent material. The carbon coating on NMC622 was achieved via polymerisation of furfuryl alcohol (FA) (≥98% (a/a), Merck KGaA, Gernsheim, Germany). Oxalic acid dihydrate (Merck KGaA, Germany) was used as the catalyst for the polycondensation reaction of furfuryl alcohol. The weight ratio of NMC622 to FA was adjusted to 6:1. The FA to oxalic acid ratio was maintained at 1 wt.% of FA, and then the NMC622 powders were dispersed in the solution of FA, followed by heating to 80 °C. Once the reactants became a gel, it was cured at 120 °C for 1 h. To obtain the carbon coating on NMC622, the cured powders were calcined at 235 °C, 300 °C, 350 °C, and 400 °C, respectively, for 2 h in air as shown in Figure S1.
Physico-Chemical Characterisation
The phase identification was carried out by powder X-ray diffraction (PXRD) using an X’Pert Pro diffractometer (Malvern/Panalytical) equipped with Cu Kα radiation (λ = 1.54060 Å). The PXRD patterns were collected between 10 and 120° (2θ) and a scan speed of 0.01061°/s. The specimen displacement, peak deconvolution, and unit cell parameters were refined by Rietveld analysis within the Highscore Plus software package. For the standard reference pattern LiNi0.65Mn0.1Co0.25O2 (98-015-9318), the correct atomic occupancy for NMC622 (Li1Ni0.60Mn0.2Co0.2O2) was considered. A scanning electron microscope SEM (Supra 40, Carl Zeiss AG, Oberkochen, Germany) was used to investigate the morphology of carbon coated and uncoated NMC622 powders. A detailed examination of the thickness and homogeneity of the coating was investigated by transmission electron microscopy TEM (TECNAI F20, FEI Company, Hillsboro, OR, USA). The elemental distribution was analysed by energy dispersive X-ray spectroscopy (EDX, EDAX Inc., Mahwah, NJ, USA). Attenuated total reflectance-Fourier-transform infrared spectroscopy (ATR-FTIR, PerkinElmer, Inc., Waltham, MA, United States) was used to determine organic functional groups. The thermal decomposition of the polymer coated NMC622 was observed using a NETZSCH STA 449 F1 thermobalance, and thermogravimetric analysis (TGA) with a differential thermal analysis sensor (DTA) coupled with a 403 Aëolos Quadro quadrupole mass spectrometer (Erich NETZSCH GmbH & Co. Holding KG, Selb, Germany) was used to determine the gas evolved during the decomposition reaction. The XPS-spectrometer (SPECS, Berlin, Germany) consists of a monochromatic (Al-Kα = 1486.6 eV) radiation source (µFocus 350) and a hemispherical energy analyzer with a wide-angle lens (PHOIBOS-150, acceptance angle: 60°, mean angle: 51° to sample surface normal). Pass energies of 100 eV and 30 eV and step widths of 0.5 eV and 50 meV were used to obtain the survey and high-resolution spectra, respectively. Measurements were conducted at 3 × 10−9 mbar, where the base pressure of the system was 1 × 10−9 mbar. To confirm the linearity of the binding energy (BE), scale methods described in ISO15472 were used. The instrument resolution was determined by measuring the Ag 3d5/2 signal of sputter cleaned silver. The data analysis was carried out by using CASA XPS software (Casa Software Ltd, UK), applying Scofield sensitivity factors [52] and transmission correction Shirley/Tougaard backgrounds [53,54]. Charge correction was carried out by shifting the adventitious carbon peak (C–C peak) to 285.0 eV binding energy. Elemental analysis (CHN) was carried out with a Perkin Elmer 2400CHN elemental analyser (PerkinElmer, Inc., Waltham, MA, USA).
Cell Preparation and Electrochemical Measurement
Pristine NMC622, carbon coated NMC622 powders as active material, in this publication called AM, and carbon black (CB) from Imerys S.A. (super-C65) were dried at 100 °C for 2 h. Manual mixing was carried out in an agate mortar. Small portions of 7 wt.% polyvinylidene fluoride (PVDF, SOLEF 5130) in N-methyl pyrrolidone (NMP) solution were added dropwise and mixed thoroughly until it formed a homogenous mixture. The dough-like mixture was transferred to a glass beaker, where the rest of the PVDF–NMP solution was added. The weight ratio of AM, CB, and PVDF was adjusted to 90:5:5 with a solid content of the slurry of 40 wt.% and stirred for 12 h. A 120 µm thick film was coated on a 16 µm aluminium foil using a doctor blade. The coating was dried at 60 °C in air for 30 min followed by 120 °C for 2 h in a vacuum oven. After this procedure, the electrode was calendared at 120 °C, where 35–40% of the porosity was retained. Electrodes were punched as 15 mm discs and subjected to another heating step at 120 °C under high vacuum for 12 h before being transferred into a glove box.
Considering a specific AM capacity of 160 mAh g−1, the average areal loading (8.5 ± 0.5 mg cm−2) of AM gave an average areal capacity of 1.4 ± 0.1 mAh cm−2. The CR2016 coin type half-cells with the NMC622 electrode active material, Celgard separator, and Li foil were assembled in an argon-filled glove box (M. BRAUN INERTGAS-SYSTEME GMBH, Garching, Germany), where H2O and O2 contents were <0.1 ppm. A volume of 100 µL of a 1M LiPF6 in ethylene carbonate (EC)):ethyl methyl carbonate (EMC) = 3:7 (w/w), and 2 wt.% vinylene carbonate (VC) (SoulBrain) solution was used as an electrolyte and added to wet the micropores of the polypropylene membrane (Celgard 2500) separators. For charge–discharge and rate capability tests, constant current constant voltage (CCCV) measurements were conducted on a Maccor Series 4000 battery tester in the voltage range of 3.0–4.3 V at 0.1 C (where 1 C = 160 mAh g−1) for the first five cycles’ formation, followed by 0.5 C for CCCV charging (CV until the current reaches 0.05 C) and 2.0 C for CC-discharging. The cyclic voltammetry (CV) was performed with a scan rate of 50 µV s−1 in the potential range of 3–4.3 V vs. Li/Li+. Electrochemical impedance spectroscopy (EIS) was carried out with an amplitude voltage of 10 mV and a frequency range of 100 mHz to 3 KHz, using a BioLogic VSP electrochemical workstation. All the electrochemical measurements were then performed at a constant temperature of 25 °C.
Conclusions
An amorphous carbon coating was successfully applied onto NMC622 materials by acid catalyst polymerisation of furfuryl alcohol followed by a calcination step for the first time. The XRD analysis showed the phase stability of NMC622 at various temperatures, SEM images showed complete coverage of NMC samples with carbon, and TEM images further confirmed the carbon coating and showed variations in the thickness of the coating as a function of the calcination temperature as well as uniformity. Additionally, XPS analysis showed the temperature dependency of the carbon coating, which was confirmed by the TEM analysis of the coated NMC samples. Furthermore, a detailed electrochemical analysis was performed to investigate the effect of the surface coating on the performance of the NMC622, and the long-term cycling of the coated samples showed improved electrochemical performance; in particular, the 15–20 nm carbon coatings showed better capacity retention and an improvement of 8% after 400 cycles. The improvement in the long-term cycling performance of the coated calcined samples shows their low electrode–electrolyte side reactions over 400 cycles. Apart from long-term performance, the coated calcined sample showed nearly 50% capacity improvement compared with the uncoated one when cycled at 10 C provided by a better percolation network. The high-rate performance improvement was further analysed by electrochemical impedance analysis, where the coated calcined samples showed lower charge-transfer resistance than the uncoated samples, which creates optimum conditions for the Li+ ions to transfer from the bulk to the surface of the electrode. Additionally, for the role of heat treatment on the NMC particles, systematic heating on the uncoated particles was carried out, showing the remaining structural stability of the NMC622 and resulting in improved capacity after 400 cycles. PXRD in post-mortem analysis showed less crystallinity for the uncoated materials compared to 400 °C calcined NMC. However, the SEM analysis showed no major difference in particle size in both materials. Hence, the optimum carbon coating without inert atmosphere improved the long-term cycling as well as the high-rate performances. Furthermore, comparing the results with the state-of-the-art reported coating materials for nickel-rich cathodes, the present coating strategy to obtain a thin coating of ~15 nm at 400 °C showed excellent capacity retention after the run of 400 complete cycles at 2 C discharge rate and a good upgrade in the C-rate performances, especially at a 10 C discharge rate, where nearly 50% enhancement was observed. In future, the study at higher potentials, in combination with state-of-the-art anodes to fabricate full cells, will help to enable high-power applications.
Supplementary Materials
The following graphs and tables are available online at https://www.mdpi.com/article/10.3390/batteries7040069/s1, Figure S1: Schematic of synthesis process; Figure S2: XPS detail spectra for (a) C 1s and (b) Ni 2p regions; Figure S3: EDAX analysis of (a) uncoated pristine NMC622, (b) Coated calcined at 235 °C, (c) coated calcined at 300 °C, (d) coated calcined at 350 °C, and (e) coated calcined at 400 °C for 120 min duration; Figure S4: Formation cycle profile of uncoated and coated calcined NMC622 sample carried at 0.1C current rate; Figure S5: XRD patterns of (a) uncoated pristine NMC622, (b) uncoated calcined at 235 °C, (c) uncoated calcined at 300 °C, (d) uncoated calcined at 350 °C, and (e) uncoated calcined at 400 °C for 120 min duration; Figure S6: Cycling performance of the uncoated NMC622 and uncoated calcined at 235 °C, 300 °C, 350 °C, and 400 °C; Figure S7: Equivalent electrical circuit for the impedance analysis of the uncoated and coated samples; Table S1: The lattice parameters of the uncoated and the coated heated NMC622 samples; Table S2: Comparison of previously reported different surface coating materials to improve their long-term electrochemical cycling performance; Table S3: Comparison of previously reported different surface coating materials to improve their high current electrochemical performances.
Author Contributions
Conceptualization, A.R.K. and J.K.; methodology, A.R.K. and J.K.; validation, J.K. and A.R.; formal analysis, A.R.K. and J.K.; investigation, A.R.K. and J.K.; resources, M.J.; data curation, A.R.K., J.K., D.L., R.H., and Y.S.; writing—original draft preparation, A.R.K. and J.K.; writing—review and editing, J.K.; A.R., D.L., M.B., J.V.M., A.H., and M.J.; visualization, A.R.K.; supervision, J.K.; project administration, J.K.; funding acquisition, M.J. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support of the Austrian Federal Ministry for Climate Action, Environment, Energy, Mobility, Innovation and Technology, without which this research would not have been possible.
Acknowledgments
We acknowledge the Analytical Instrumentation Center of the Technische Universität Wien for providing XPS analysis and Xiaoxue Lu, Xinhua Zhu, and Mohammad Furquan for the scientific discussion. Special thanks to Jacqueline Winter for the review and language editing of the manuscript.
References
Noh, H.-J.; Youn, S.; Yoon, C.S.; Sun, Y.K. Comparison of the structural and electrochemical properties of layered Li [NixCoyMnz] O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 2013, 233, 121–130. [Google Scholar] [CrossRef]
Lin, F.; Markus, I.M.; Nordlund, D.; Weng, T.C.; Asta, M.D.; Xin, H.L.; Doeff, M.M. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 2014, 5, 3529. [Google Scholar] [CrossRef] [PubMed]
Manthiram, A.; Song, B.; Li, W. A perspective on nickel-rich layered oxide cathodes for lithium-ion batteries. Energy Storage Mater. 2017, 6, 125–139. [Google Scholar] [CrossRef]
Myung, S.-T.; Maglia, F.; Park, K.-J.; Yoon, C.S.; Lamp, P.; Kim, S.-J.; Sun, Y.-K. Nickel-rich layered cathode materials for automotive lithium-ion batteries: Achievements and perspectives. ACS Energy Lett. 2017, 2, 196–223. [Google Scholar] [CrossRef]
Schipper, F.; Erickson, E.M.; Erk, C.; Shin, J.-Y.; Chesneau, F.F.; Aurbach, D. Review—Recent Advances and Remaining Challenges for Lithium Ion Battery Cathodes. J. Electrochem. Soc. 2016, 164, A6220–A6228. [Google Scholar] [CrossRef]
Kim, J.; Lee, H.; Cha, H.; Yoon, M.; Park, M.; Cho, J. Prospect and reality of Ni-rich cathode for commercialization. Adv. Energy Mater. 2018, 8, 1–25. [Google Scholar] [CrossRef]
Liu, W.; Oh, P.; Liu, X.; Lee, M.J.; Cho, W.; Chae, S.; Kim, Y.; Cho, J. Nickel-rich layered lithium transition-metal oxide for high-energy lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 4440–4457. [Google Scholar] [CrossRef] [PubMed]
Herzog, M.J.; Gauquelin, N.; Esken, D.; Verbeeck, J.; Janek, J. Increased Performance Improvement of Lithium-Ion Batteries by Dry Powder Coating of High-Nickel NMC with Nanostructured Fumed Ternary Lithium Metal Oxides. ACS Appl. Energy Mater. 2021, 4, 8832–8848. [Google Scholar] [CrossRef]
Li, T.; Yuan, X.-Z.; Zhang, L.; Song, D.; Shi, K.; Bock, C. Degradation mechanisms and mitigation strategies of nickel-rich NMC-based lithium-ion batteries. Electrochem. Energy Rev. 2019, 31, 43–80. [Google Scholar] [CrossRef]
Zhu, W.; Huang, X.; Liu, T.; Xie, Z.; Wang, Y.; Tian, K.; Bu, L.; Wang, H.; Gao, L.; Zhao, J. Ultrathin Al2O3 Coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced cycleability at extended voltage ranges. Coatings 2019, 9, 92. [Google Scholar] [CrossRef]
Wang, J.; Du, C.; Yan, C.; He, X.; Song, B.; Yin, G.; Zuo, P.; Cheng, X. Al2O3 coated concentration-gradient Li[Ni0.73Co0.12Mn0.15]O2 cathode material by freeze drying for long-life lithium ion batteries. Electrochim. Acta 2015, 174, 1185–1191. [Google Scholar] [CrossRef]
Jian, Z.; Wang, W.; Wang, M.; Wang, Y.; AuYeung, N.; Liu, M.; Feng, Z. Al2O3 coated LiCoO2 as cathode for high-capacity and long-cycling Li-ion batteries. Chin. Chem. Lett. 2018, 29, 1768–1772. [Google Scholar] [CrossRef]
Schipper, F.; Bouzaglo, H.; Dixit, M.; Erickson, E.M.; Weigel, T.; Talianker, M.; Grinblat, J.; Burstein, L.; Schmidt, M.; Lampert, J.; et al. From surface ZrO2 coating to bulk Zr doping by high temperature annealing of nickel-rich lithiated oxides and their enhanced electrochemical performance in lithium ion batteries. Adv. Energy Mater. 2018, 8, 1701682. [Google Scholar] [CrossRef]
Tao, T.; Chen, C.; Yao, Y.; Liang, B.; Lu, S.; Chen, Y. Enhanced electrochemical performance of ZrO2 modified LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries. Ceram. Int. 2017, 43, 15173–15178. [Google Scholar] [CrossRef]
Laskar, M.R.; Jackson, D.H.K.; Xu, S.; Hamers, R.J.; Morgan, D.; Kuech, T.F. Atomic layer deposited MgO: A lower overpotential coating for Li[Ni0.5Mn0.3Co0.2]O2 cathode. ACS Appl. Mater. Interfaces 2017, 9, 11231–11239. [Google Scholar] [CrossRef]
Zhang, H.; Xu, J.; Zhang, J. Surface-coated LiNi0.8Co0.1Mn0.1O2 (NCM811) cathode materials by Al2O3, ZrO2, and Li2O-2B2O3 thin-layers for improving the performance of lithium ion batteries. Front. Mater. 2019, 6, 309. [Google Scholar] [CrossRef]
Hildebrand, S.; Vollmer, C.; Winter, M.; Schappacher, F.M. Al2O3, SiO2 and TiO2 as coatings for safer LiNi0.8Co0.15Al0.05O2 cathodes: Electrochemical performance and thermal analysis by accelerating rate calorimetry. J. Electrochem. Soc. 2017, 164, A2190–A2198. [Google Scholar] [CrossRef]
Lu, X.; Zhang, N.; Jahn, M.; Pfleging, W.; Seifert, H.J. Improved capacity retention of SiO2 -coated lithium-ion batteries. Appl. Sci. 2019, 9, 3671. [Google Scholar] [CrossRef]
Kong, J.-Z.; Ren, C.; Tai, G.-A.; Zhang, X.; Li, A.-D.; Wu, D.; Li, H.; Zhou, F. Ultrathin ZnO coating for improved electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material. J. Power Sources 2014, 266, 433–439. [Google Scholar] [CrossRef]
Xie, Z.; Zhang, Y.; Yuan, A.; Xu, J. Effects of lithium excess and SnO2 surface coating on the electrochemical performance of LiNi0.8Co0.15Al0.05O2 cathode material for Li-ion batteries. J. Alloys Compd. 2019, 787, 429–439. [Google Scholar] [CrossRef]
Loghavi, M.M.; Mohammadi-Manesh, H.; Eqra, R. Y2O3-decorated LiNi0.8Co0.15Al0.05O2 cathode material with improved electrochemical performance for lithium-ion batteries. J. Electroanal. Chem. 2019, 848, 113326. [Google Scholar] [CrossRef]
Li, X.; Jin, L.; Song, D.; Zhang, H.; Shi, X.; Wang, Z.; Zhang, L.; Zhu, L. LiNbO3-coated LiNi0.8Co0.1Mn0.1O2 cathode with high discharge capacity and rate performance for all-solid-state lithium battery. J. Energy Chem. 2020, 40, 39–45. [Google Scholar] [CrossRef]
Shen, B.; Liu, Q.; Wang, L.; Yin, G.; Zuo, P.; Ma, Y.; Cheng, X.; Du, C.; Gao, Y. Mixed lithium ion and electron conducting LiAlPO3.93F1.07-coated LiCoO2 cathode with improved electrochemical performance. Electrochem. commun. 2017, 83, 106–109. [Google Scholar] [CrossRef]
Chen, X.; Ma, F.; Li, Y.; Liang, J.; Matthews, B.; Sokolowski, J.; Han, J.; Wu, G.; Lu, X.; Li, Q. Nitrogen-doped carbon coated LiNi0.6Co0.2Mn0.2O2 cathode with enhanced electrochemical performance for Li-Ion batteries. Electrochim. Acta 2018, 284, 526–533. [Google Scholar] [CrossRef]
Mauger, A.; Julien, C. Surface modifications of electrode materials for lithium-ion batteries: Status and trends. Ionics 2014, 20, 751–787. [Google Scholar] [CrossRef]
Chen, Z.; Zhang, Z.; Liu, P.; Wang, S.; Zhang, W.; Chen, D. Facile preparation of carbon-LiNi1/3Co1/3Mn1/3O2 with enhanced stability and rate capability for lithium-ion batteries. J. Alloys Compd. 2019, 780, 643–652. [Google Scholar] [CrossRef]
Guo, R.; Shi, P.; Cheng, X.; Du, C. Synthesis and characterization of carbon-coated LiNi1/3Co1/3Mn1/3O2 cathode material prepared by polyvinyl alcohol pyrolysis route. J. Alloys Compd. 2009, 473, 53–59. [Google Scholar] [CrossRef]
Dominko, R.; Bele, M.; Gaberscek, M.; Remskar, M.; Hanzel, D.; Pejovnik, S.; Jamnik, J. Impact of the carbon coating thickness on the electrochemical performance of LiFePO4/C composites. J. Electrochem. Soc. 2005, 152, A607–A610. [Google Scholar] [CrossRef]
Sides, C.R.; Croce, F.; Young, V.Y.; Martin, C.R.; Scrosati, B. A high-rate, nanocomposite LiFePO4/carbon cathode. Electrochem. Solid-State Lett. 2005, 8, 484–487. [Google Scholar] [CrossRef]
Singh, S.; Raj, A.K.; Sen, R.; Johari, P.; Mitra, S. Impact of Cl doping on electrochemical performance in orthosilicate (Li2FeSiO4): A density functional theory supported experimental approach. ACS Appl. Mater. Interfaces 2017, 9, 26885–26896. [Google Scholar] [CrossRef]
Singh, S.; Panda, M.R.; Sen, R.; Johari, P.; Sinha, A.K.; Meena, S.S.; Mitra, S. Study of higher discharge capacity, phase transition, and relative structural stability in Li2FeSiO4 cathode upon lithium extraction using an experimental and theoretical approach and full cell prototype study. ACS Appl. Energy Mater. 2019, 2, 6584–6598. [Google Scholar] [CrossRef]
Wang, L.-P.; Zhang, X.-D.; Wang, T.-S.; Yin, Y.-X.; Shi, J.-L.; Wang, C.-R.; Guo, Y.-G. Ameliorating the interfacial problems of cathode and solid-state electrolytes by interface modification of functional polymers. Adv. Energy Mater. 2018, 8, 1801528. [Google Scholar] [CrossRef]
Babbar, P.; Niehoff, P.; Schappacher, F.; Winter, M. Studying the effects of carbon coatings on the electrochemical performance of LiNi1/3Co1/3Mn1/3O2. Meet. Abstr. 2018, MA2018-01, 359. [Google Scholar]
Sim, S.-J.; Lee, S.-H.; Jin, B.-S.; Kim, H.-S. Use of carbon coating on LiNi0.8Co0.1Mn0.1O2 cathode material for enhanced performances of lithium-ion batteries. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
Kong, L.; Guan, H.; Wang, X. In situ polymerization of furfuryl alcohol with ammonium dihydrogen phosphate in poplar wood for improved dimensional stability and flame retardancy. ACS Sustain. Chem. Eng. 2018, 6, 3349–3357. [Google Scholar] [CrossRef]
Lascovich, J.C.; Giorgi, R.; Scaglione, S. Evaluation of the sp2/sp3 ratio in amorphous carbon structure by XPS and XAES. Appl. Surf. Sci. 1991, 47, 17–21. [Google Scholar] [CrossRef]
Tondi, G.; Cefarin, N.; Sepperer, T.; D’Amico, F.; Berger, R.J.F.; Musso, M.; Birarda, G.; Reyer, A.; Schnabel, T.; Vaccari, L. Understanding the polymerization of poly-furfuryl alcohol: Ring opening and diels-alder reactions. Polymers 2019, 11, 2126. [Google Scholar] [CrossRef]
Janus, R.; Wach, A.; Kuśtrowski, P.; Dudek, B.; Drozdek, M.; Silvestre-Albero, A.M.; Rodríguez-Reinoso, F.; Cool, P. Investigation on the low-temperature transformations of poly (furfuryl alcohol) deposited on MCM-41. Langmuir 2013, 29, 3045–3053. [Google Scholar] [CrossRef]
Zhang, X.; Jiang, W.J.; Mauger, A.; Qilu; Gendron, F.; Julien, C.M. Minimization of the cation mixing in Li1+x(NMC)1-xO2 as cathode material. J. Power Sources 2010, 195, 1292–1301. [Google Scholar] [CrossRef]
Lu, Z.; Beaulieu, L.Y.; Donaberger, R.A.; Thomas, C.L.; Dahn, J.R. Synthesis structure, and electrochemical behavior of Li [NixLi1/3-2x/3Mn2/3-x/3]O2. J. Electrochem. Soc. 2002, 149, A778–A791. [Google Scholar] [CrossRef]
Julien, C. Local cationic environment in lithium nickel–cobalt oxides used as cathode materials for lithium batteries. Solid State Ionics 2000, 136–137, 887–896. [Google Scholar] [CrossRef]
Kosova, N.V.; Devyatkina, E.T. Comparative study of LiCoO2 surface modified with different oxides. J. Power Sources 2007, 174, 959–964. [Google Scholar] [CrossRef]
Choura, M.; Belgacem, N.M.; Gandini, A. Acid-catalyzed polycondensation of furfuryl alcohol: Mechanisms of chromophore formation and cross-linking. Macromolecules 1996, 29, 3839–3850. [Google Scholar] [CrossRef]
Verdejo, R.; Lamoriniere, S.; Cottam, B.; Bismarck, A.; Shaffer, M. Removal of oxidation debris from multi-walled carbon nanotubes. Chem. Commun. 2007, 106, 513–515. [Google Scholar] [CrossRef] [PubMed]
Branca, C.; Corsaro, C.; Frusteri, F.; Magazù, V.; Mangione, A.; Migliardo, F.; Wanderlingh, U. Structural and vibrational properties of carbon nanotubes by TEM and infrared spectroscopy. Diam. Relat. Mater. 2004, 13, 1249–1253. [Google Scholar] [CrossRef]
Bertarione, S.; Bonino, F.; Cesano, F.; Jain, S.; Zanetti, M.; Scarano, D.; Zecchina, A. Micro-FTIR and micro-raman studies of a carbon film prepared from furfuryl alcohol polymerization. J. Phys. Chem. B 2009, 113, 10571–10574. [Google Scholar] [CrossRef] [PubMed]
Singh, S.; Mitra, S. Improved electrochemical activity of nanostructured Li2FeSiO4/MWCNTs composite cathode. Electrochim. Acta 2014, 123, 378–386. [Google Scholar] [CrossRef]
De Almeida Filho, C.; Zarbin, A.J.G. Porous carbon obtained by the pyrolysis of TiO2/poly (furfuryl alcohol) nanocomposite: Preparation, characterization and utilization for adsorption of reactive dyes from aqueous solution. J. Braz. Chem. Soc. 2006, 17, 1151–1157. [Google Scholar] [CrossRef]
Czigány, Z.; Brunell, I.F.; Neidhardt, J.; Hultman, L.; Suenaga, K. Growth of fullerene-like carbon nitride thin solid films consisting of cross-linked nano-onions. Appl. Phys. Lett. 2001, 79, 2639–2641. [Google Scholar] [CrossRef]
Wang, C.; Appleby, A.J.; Little, F.E. Electrochemical impedance study of initial lithium ion intercalation into graphite powders. Electrochim. Acta 2001, 46, 1793–1813. [Google Scholar] [CrossRef]
Xu, K.; Lam, Y.; Zhang, S.S.; Jow, T.R.; Curtis, T.B. Solvation Sheath of Li + in nonaqueous electrolytes and its implication of graphite/electrolyte interface chemistry. J. Phys. Chem. C 2007, 111, 7411–7421. [Google Scholar] [CrossRef]
Scofield, J.H. Hartree-Slater subshell photoionization cross-sections at 1254 and 1487 eV. J. Electron Spectros. Relat. Phenom. 1976, 8, 129–137. [Google Scholar] [CrossRef]
Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
Tougaard, S. Universality classes of inelastic electron scattering cross-sections. Surf. Interface Anal. 1997, 25, 137–154. [Google Scholar] [CrossRef]
StrategX Elements: Targeting Underexplored Regions in Northern Canada for Energy TransitionMetals
Originally posted on Investingnews.com
StrategX Elements Corp. (CNSX:STGX) focuses on discovering new energy transition metal deposits required for the shift to clean and sustainable energy technologies. The company’s assets centers on cobalt, nickel, and other energy transition metals to contribute to Canada’s domestic supply chain. StrategX’s five 100-percent-owned assets are within Nunavut and the Northwest Territories.
The company’s assets cover 110,00 hectares in the Northwest Territories and 60,000 hectares in Nunavut. StrategX’s flagship project, Nagvaak in Nunavut, is highly prospective for nickel, vanadium, cobalt, copper, molybdenum, silver and PGMs. In Q1 2023, the company plans to conduct a first phase 2,000-meter drill program to confirm suspected deposits. The company’s additional projects are moving towards exploratory drilling as exploration campaigns aim to identify priority targets.
StrategX believes in the importance of maintaining a positive ESGI rating as a company focusing on supporting renewable energy. Leadership has prioritized building solid relationships with local communities from the beginning and integrating these communities into the project. The company understands that communities need to be involved in unlocking the critical mineral potential of its assets.
Company Highlights
StrategX is a Canadian exploration company with assets in Nunavut and the Northwest Territories focusing on making discoveries of energy metals, including cobalt, nickel, vanadium, and PGEs.
The company aims to contribute to Canada’s emerging critical metal supply chain to support net-zero initiatives.
StrategX has five 100-percent-owned projects in Nunavut and the Northwest Territories targeting metals critical to transitioning to clean energy technologies.
The Nagvaak project, the company’s flagship asset, is scheduled to begin exploratory drilling in Q1 2023 to follow up on priority targets identified in the Q3-Q4 2022 campaign.
A strong emphasis is placed on achieving a positive ESGI rating, and management has prioritized building relationships with local communities from the beginning.
A management team with expertise throughout the natural resources industry and a track record of success lead the company toward its goals.
This StrategX Elements Corp profile is part of a paid investor education campaign.*
Click here to connect with StrategX Elements Corp. (CNSX:STGX) to receive an Investor Presentation
Solid-state EV battery stays in shape to hint at huge range extensions
Originally posted on Newatlas.com
A new electrode material developed for EV batteries could unlock faster-charging and greater range
Solid-state batteries, where the electrolyte consists of a solid material rather than a liquid one, hold massive potential for future energy storage applications, but have been plagued by stability issues that impact their longevity. A new design points to a new solution to this problem, with the team developing a novel electrode material that retains its volume throughout charging to enable the battery to endure hundreds of cycles.
An ability to operate an electric vehicle or smartphone on a solid-state battery instead of today’s lithium-ion architecture could see them run for longer before they need recharging, and require less plug-in time when they do. Their stability issues, however, are related to the movement of lithium ions in and out of the battery electrodes during charging, which causes them to expand and shrink and ultimately alters the chemistry of the device.
An international team of scientists has designed a new electrode for these batteries that is claimed to offer unprecedented stability. The material is made of lithium titanate and lithium vanadium dioxide, ground down into nanometer-sized particles. Used as a battery electrode, this material offers high-capacity, and enables lithium ions to be reversibly inserted and extracted during charging and discharging.
The result is an electrode material that retains the same volume during operation. The scientists have pinned this on a delicate balancing act that takes place as lithium ions exit and vanadium ions migrate from their original positions to fill in these empty spaces.
“When shrinkage and expansion are well balanced, dimensional stability is retained while the battery is charged or discharged, i.e. during cycling,” said Professor Naoaki Yabuuchi of Yokohama National University, Japan, who led the research. “We anticipate that a truly dimensionally invariable material – one that retains its volume upon electrochemical cycling – could be developed by further optimizing the chemical composition of the electrolyte.”
The novel electrode material was put to the test in a solid-state battery cell and performed impressively, with a high capacity of 300 mAh/g, and perhaps most importantly, no degradation over 400 charge and discharge cycles.
“The absence of capacity fading over 400 cycles clearly indicates the superior performance of this material compared with those reported for conventional all-solid-state cells with layered materials," said study author Associate Professor Neeraj Sharma.
The team plans to continue refining the electrolyte to build the kinds of batteries needed to serve electric vehicles, with greater safety and lifespan. This could mean not just electric vehicles the way we see them today, but helping usher in a new era where they charge much faster and cover greater distances.
"This finding could drastically reduce battery costs,” said Yabuuchi. “The development of practical high-performance solid-state batteries can also lead to the development of advanced electric vehicles. In the future, for instance, it may be possible to fully charge an electric vehicle in as little as five minutes.”
The research was published in the journal Nature Materials.
Graphite deficit starting this year, as demand for graphite battery anode ingredient exceeds supply
Originally posted on Aheadoftheherd.com
Although EV market share is still tiny compared to traditional vehicles, that is likely to change in the coming years as major economies transition away from fossil fuels and move into clean energy.
US President Joe Biden has signed an executive order requiring that half of all new vehicle sales be electric by 2030. China, the world’s biggest EV market, has a similar mandate that requires electric cars to make up 40% of all sales. The European Union is also seeking to have at least 30 million zero-emission vehicles on its roads by then.
According to the IEA’s Global Electric Vehicle Outlook, if governments are able to ramp up their efforts to meet energy and climate goals, the global electric vehicle fleet could reach as high as 230 million by the end of the decade, compared to about 20 million currently.
With more electric cars comes the need for more raw materials like lithium, nickel and graphite to build batteries. The IEA believes mineral demand for use in EVs and battery storage must grow at least 30 times by 2040 to meet various climate goals.
Fastmarkets forecast that EV sales will experience a compound annual growth rate of 40% per year through 2025, when EV penetration is expected to reach 15%. After that, EV market share is expected to rise further, reaching 35% by 2030.
One mineral that has been overlooked, but is an essential part of vehicle electrification, is graphite.
At AOTH, we believe graphite represents a “backdoor” market opportunity brought about by the clean energy transition. This is for several reasons:
Graphite as anode material
The lithium-ion battery used to power electric vehicles is made of two electrodes — an anode (negative) on one side and a cathode (positive) on the other. At the moment, graphite is the only material that can be used in the anode, there are no substitutes.
This is due to the fact that, with high natural strength and stiffness, graphite is an excellent conductor of heat and electricity. Being the only other natural form of carbon besides diamonds, it is also stable over a wide range of temperatures.
The cathode is where metals like lithium, nickel, manganese and cobalt are used. Depending on the battery chemistry, there are different options available to battery makers (see below).
Graphite is thus considered indispensable to the global shift towards electric vehicles. It is also the largest component in lithium-ion batteries by weight, with each battery containing 20-30% graphite. But due to losses in the manufacturing process, it actually takes 30 times more graphite than lithium to make the batteries.
According to the World Bank, graphite accounts for nearly 53.8% of the mineral demand in batteries, the most of any. Lithium, despite being a staple across all batteries, accounts for only 4% of demand.
An electric car contains more than 200 pounds (>90 kg) of coated spherical purified graphite (CSPG), meaning it takes 10 to 15 times more graphite than lithium to make a Li-ion battery.
Graphite is so essential to a lithium battery, that Tesla’s Elon Musk famously said, “Our cells should be called Nickel-Graphite, because primarily the cathode is nickel and the anode side is graphite with silicon oxide.”
Demand overflow
The anode material, called spherical graphite, is manufactured from either flake graphite concentrates produced from graphite mines, or from synthetic/artificial graphite. Only flake graphite upgraded to 99.95% purity can be used.
An average plug-in EV has 70 kg of graphite, or 10 kg for a hybrid. Every 1 million EVs requires about 75,000 tonnes of natural graphite, equivalent to a 10% increase in flake graphite demand.
According to Benchmark Mineral Intelligence (BMI), the flake graphite feedstock required to supply the world’s lithium-ion anode market is projected to reach 1.25 million tonnes per annum by 2025. The amount of mined graphite for all uses in 2021, was just 1 million tonnes. (USGS)
Furthermore, the London-based price reporting agency forecasts demand for graphite from the battery anode segment could increase by seven times in the next decade as the growth in EV sales continues to drive construction of lithium-ion megafactories.
BloombergNEF expects demand for battery minerals to remain robust through 2030, with graphite demand increasing four-fold.
The International Energy Agency (IEA) goes 10 years further out, predicting that growth in demand for selected minerals from clean energy technologies by scenario, 2020 relative to 2040, will see: increases of lithium 13x to 42x, graphite 8x to 25x, cobalt 6x to 21x, nickel 7x to 19x, manganese 3x to 8x, rare earths 3x to 7x, and copper 2x to 3x.
Source: UBS
Source: IEA
Supply squeeze
As vehicle electrification continues, and few new sources are discovered worldwide, BMI estimates the graphite market could reach a deficit as early as this year, with the supply shortfall growing to 8Mt by 2040; to fill this gap, the mining industry would need to produce nearly 8x as much graphite as it does currently, over the next 18 years.
Source: BMI
On June 7, in an article titled ‘How a battery metals squeeze puts EV future at risk’, The Washington Post reported, Factory lines churning out power packs to fuel a clean energy future are being built faster than strained supply chains can keep up. A global rush to lock in stocks of lithium, nickel, cobalt and other key ingredients from a handful of nations has sent prices hurtling higher… While factories can be built in about 18 months, mines can typically take seven years or longer to come online.
June saw repeated concerns over the supply of battery metals forecast for the decade ahead, including from Tesla. CEO Elon Musk reportedly explained that production has been hindered by raw material shortages and shutdowns of assembly lines in China.
Lack of diverse supply
Almost all graphite processing today takes place in China because of the ready availability of graphite there, weak environmental standards and low costs. Nearly 60% of the world’s mined production last year also came from China, making it a dominant player in every stage of the graphite supply chain.
After China, the next leading graphite producers are Mozambique, Brazil, Madagascar, Canada and India. The US does not produce any natural graphite, therefore it must rely solely on imports to satisfy domestic demand.
Data source: USGS. Image by Visual Capitalist
The level of foreign dependence has increased over the years. The US imported 38,900 tonnes of graphite in 2016, then peaking at 70,700 tonnes in 2018.
The latest publication from the USGS shows that imports in 2021 totaled 53,000 tonnes, of which 71% was high-purity flake graphite, 42% was amorphous, and 1% was lump and chip graphite.
The main import sources were China (33%), Mexico (21%), Canada (17%) and India (9%).
Since China controls all spherical graphite processing, the US is not actually 33% dependent on China for its battery-grade graphite, but 100%.
This is why the US government has included graphite among the 35 minerals that it deems “critical to its national security and economy.”
A White House report on critical supply chains showed that graphite demand for clean energy applications will require 25 times more graphite by 2040 than was produced worldwide in 2020.
Graphite pricing
The value of natural graphite has increased significantly over the course of the past year, with demand continuing to outstrip supply. According to Benchmark Mineral Intelligence, prices have gone up steadily since January 2021 on all types of graphite, with fines increasing 44.50% from USD$500/ton in January of 2021 to $723/t in May of 2022. Using those same dates, large flake graphite prices climbed 19.85% from $983/t to $1,187/t, and spherical graphite rose 8.39% from $2,958/t to $3,207/t.
More recently, flake and spherical graphite prices are both up slightly. According to Fastmarkets, for the week ending June 16, the spot price of China flake graphite 194 EXW was up 0.37% over 30 days, and 19.39% over 360 days. Graphite produced at 94-97% purity is considered best suited for batteries, before it is upgraded to 99.99% purity to make spherical graphite. Spherical graphite 99.95% min EXW China was up 1.58% over the past 30 days, for the week ended June 16.
China flake graphite 94% C (-100 mesh) was priced at $830 per ton, with Europe flake graphite of the same grade and size selling for $920/t.
Source: Fastmarkets
Conclusion
During a time of price weakness for a number of industrial metals (copper, zinc, aluminum, for example), the price of graphite, being critical to the electric-vehicle transition, has held up extremely well.
Flake and spherical graphite are both trending higher, in fact the prices of all types of graphite (fines, large flake, spherical) have increased significantly since January 2021, on the back of robust demand from battery-makers and EV manufacturers, and limited supply.
According to BMI, in 2022 demand for lithium-ion batteries is growing at its fastest ever, on course for a year-on-year growth rate of nearly 50%.
Source: BMI
While this will increase the need for other battery minerals, such as lithium, nickel and cobalt, graphite remains the highest-intensity mineral in the lithium-ion battery by weight, with over 570,000 tonnes of natural flake to be consumed in 2022.
Yet as Seeking Alpha observes, consumer demand for electric vehicles surpasses our ability to supply them. Waiting times for EVs are lengthening, a lithium ion battery shortage is hitting many automakers, and, most crucially, key raw material prices are at all-time highs.
This bodes well for companies with large graphite deposits in safe jurisdictions, that can not only capitalize on high prices, but contribute to the local graphite supply chain and lessen the dependence on China for graphite mining and especially, graphite processing.
For years neglected by governments, critical minerals like graphite are finally getting the attention they deserve. In June, the Canadian government unveiled its low-carbon industrial strategy, that will see Ottawa partnering with each province to “identify, prioritize and pursue opportunities”. Specific to critical minerals, this means battery manufacturing in Quebec and electric vehicle production in Ontario.
Natural Resources Minister Jonathan Wilkinson pointed to CAD$3.8 billion already earmarked for critical minerals in the April budget. On top of that, “we have a billion and a half dollars in the Clean Fuels Fund, we have eight billion dollars in the Net Zero Accelerator, we’re setting up the Clean Growth Fund, we have the Canada Infrastructure Bank,” Bloomberg quoted him saying. He added:
“The average mine takes 15 years to bring into production. In the context of the energy transition, we don’t have 15 years if we’re actually going to provide enough of the minerals to be able to support just the battery development. So it behooves us to bring everybody into the room to figure out how to do it.”
At AOTH, we couldn’t agree more. Canada’s new industrial strategy dovetails with what is happening south of the border.
The US, which has long sought to improve its battery supply chain, recently invoked its Cold War powers by including lithium, nickel, cobalt, graphite and manganese on the list of items covered by the 1950 Defense Production Act, previously used by President Harry Truman to make steel for the Korean War.
To bolster domestic production of these minerals, US miners can now access $750 million under the act’s Title III fund, which can be used for current operations, productivity and safety upgrades, and feasibility studies. The DPA could also cover the recycling of these materials.
Later this year, the Department of Energy will begin doling out $6 billion in grants for battery production, half of which are earmarked for domestic supplies of materials and battery recycling.
The Biden administration has already allocated $6 billion as part of the $1.2 trillion infrastructure bill, towards developing a reliable battery supply chain and weaning the auto industry off its reliance on China, the biggest EV market and leading producer of lithium-ion cells.
Among the minerals key to winning the global EV race, graphite arguably is most significant and should be a top priority for the US, given it is the essential ingredient in electric vehicle batteries.
A global graphite shortage is a matter of when, not if, without new sources of supply. For the US, which is 100% dependent on foreign imports of the material, it’s a ticking time bomb that could completely derail the nation’s vehicle electrification and decarbonization ambitions.
This all goes back to the importance of establishing a reliable, secure and sustainable “mine to battery” EV supply chain, beginning with a domestic graphite source and integrating it with processing, manufacturing and recycling to create a full circular economy.
More graphite needed for EVs
Originally posted on Metaltechnews.com
Core from drilling a high-grade lens at Graphite Creek, a world-class deposit in Alaska that is the first link of Graphite One's proposed lithium-ion battery anode material supply chain.
By 2030, batteries will likely need more than 5x all the graphite mined in 2021
While shortages of the lithium and nickel needed for electric vehicle batteries has dominated news headlines over the past year, the massive demand for graphite has largely been overlooked. As the primary ingredient in the anode side of lithium-ion batteries, graphite is the single largest element in lithium-ion batteries and the mining sector's inability to keep pace with skyrocketing demand of this critical mineral could put the brakes on the EV revolution.
According to the global lithium-ion battery supply chain experts at Benchmark Mineral Intelligence, a megafactory capable of producing 30 gigawatt-hours of battery storage annually requires about 33,000 metric tons of graphite per year.
When you extrapolate this out over the more than 300 gigafactories that are being built or are in the pipeline, this equates to up to 9.9 million metric tons of graphite per year to feed all these lithium-ion battery plants running at full capacity. Using the 70-70 rule – a more realistic measure that 70% of these battery plants go into production running at an average of 70% design capacity – the global lithium battery sector would need about 4.9 million metric tons of graphite per year.
This correlates with S&P Global Platts' forecast that by 2030 it will take 5 million to 6 million metric tons of graphite to meet annual global demand for this critical carbon material.
This compares to only about 1 million metric tons that was mined globally to meet the demands of all industrial sectors during 2021, according to "Mineral Commodity Summaries 2022," an annual report published by the United States Geological Survey.
Ramping up graphite production by 500 to 600% over 10 years is an enormous task for global miners.
"Supply will struggle to catch up with graphite demand," said George Miller, senior price analyst at Benchmark.
Emerging NA supply chain
Currently, China dominates both the mining of graphite and upgrading this carbon material into the coated spherical graphite that is packed into the anodes of lithium-ion batteries.
According to USGS, the Middle Kingdom accounted for 82% of the world's mined graphite last year and produced nearly 100% of the battery-grade anode material.
With only limited supplies of graphite currently being mined in Canada and Mexico, and graphite anode material production at its earliest stages in the U.S., North American automakers are nearly 100% dependent on imports from China for this increasingly competitive product.
"North America produced only 1.2% of the world's graphite supply with production in Canada and Mexico," USGS inked in its 2022 minerals report. "Two companies were developing graphite mining projects in the United States-one in Alabama and one in Alaska."
Both these companies – Westwater Resources Inc. in Alabama and Graphite One Inc. in Alaska – have plans to develop both graphite mines and the processing facilities to produce the spherical graphite that serves as the anode material in most lithium-ion batteries.
Syrah Resources Ltd. is also scaling up the production of battery-grade anode materials at Vidalia, a facility in Louisiana that has attracted the attention of both Tesla Inc. and the U.S. Department of Energy.
Tesla entered into a deal to buy coated spherical graphite produced at Vidalia, and DOE has loaned Syrah $102.1 million to fund the expansion of this active anode material in Louisiana.
"Projects like Syrah Vidalia are critical to our national security, our foreign policy, building our supply chain, and our economy," said DOE Loan Programs Office Director Jigar Shah.
Ford Motor Company has also entered into a deal to off-take graphite from Syrah's Vidalia facility.
In Canada, Nouveau Monde Graphite Inc. is advancing a complete supply chain to provide battery and EV manufacturers with zero-carbon graphite anode material from the hydro-powered mine and processing facilities it is developing in Quebec.
"We are making significant progress on our objectives at a time when the market is feeling the pressure of limited supply options, rising prices and complicated logistics," Nouveau Monde Graphite Chair Arne Frandsen said in March.
Alabama graphite
Alabama is rapidly emerging as a hub for the production of coated spherical graphite and the EVs that are driving enormous new demand for this anode material.
In April, Alabama Gov. Kay Ivey and Congressman Mike Rogers joined other state and local government policymakers and business leaders to break ground on a $202 million coated spherical graphite processing facility being developed by Westwater.
"Alabama, which is home to Mercedes-Benz, Honda, Hyundai, Toyota and Mazda, is among the top four states in the nation in automobile production," said Gov. Ivey. "This plant will make Alabama an even bigger player in the fast-growing electric vehicle sector."
The Mercedes EQS EV being produced in Alabama needs roughly 250 pounds of graphite for each 107.8-kilowatt-hour battery that provides this luxury sedan with an impressive 350 miles of range on a single charge.
While EV battery sizes vary by make and model, the Mercedes EQS represents a middle ground between more economical cars like the standard range Tesla Model 3, which needs about 120 lb of graphite for its battery, and full-size SUVs like the GMC Hummer, which requires roughly 500 lb of this anode ingredient.
With every major automaker on Earth electrifying their vehicles, there is massive new demand for the coated spherical graphite that will soon be produced at the Westwater facility in Alabama.
Alabama Graphite Products, a Westwater subsidiary, will use a proprietary process to purify raw graphite and refine it into battery-grade anode material.
Westwater says this process is safer and more environmentally friendly than the hydrofluoric acid-based process commonly used in China and elsewhere.
The initial phase of this processing plant, which is slated for completion by mid-2023, is designed to produce 7,500 metric tons of refined graphite.
"The construction of this plant is the result of a lot of work, cooperation, planning and vision by numerous people over a number of years," said Chad Potter, President and CEO of Westwater Resources and Alabama Graphite Products.
Last year, Gov. Ivey signed an incentive package that will provide Alabama Graphite Products with $29.9 million in jobs and tax credits over 15 years and $925,000 in job training and employee recruitment incentives for a facility.
"The cooperation and assistance we have received – from tax incentives to utilities to workforce development – has been incredible. We would not be here today without their support," Potter added.
In the beginning, the feedstock for the Alabama Graphite refinery will be imported, but Westwater intends to develop a mine at its Coosa project in the famed Alabama Graphite Belt by 2028.
According to a 2015 estimate, Coosa hosts 78.5 million metric tons of indicated resource averaging 2.39% (1.9 million metric tons) graphite.
Establishing a mine at Coosa would provide a much-needed local supply of graphite for the Alabama refinery and a new source of jobs for residents of Coosa County.
"As our investment of millions of dollars and our commitment to invest even more indicate, we are firmly committed to Alabama and this community, and we look forward to being here for many years to come," said Potter.
Alaska graphite
Much like Westwater, Graphite One Inc. is looking to establish a complete graphite anode material supply chain in the U.S., which would involve developing a mine at its enormous Graphite Creek deposit in Alaska that would provide the primary feed for a battery material processing and recycling facility in the state of Washington.
Located along Alaska's west coast about 50 miles north of the legendary gold mining town of Nome, Graphite Creek hosts 32.5 million metric tons of measured and indicated resources averaging 5.25% (1.7 million metric tons) graphite, plus 254.7 million metric tons of inferred resource averaging 5.11% (13 million metric tons) graphite.
A prefeasibility study finalized in August envisions a mine at Graphite Creek that would produce an average of 51,813 metric tons of graphite concentrate per year, which would be shipped to the company's planned facility in Washington where low-cost and low-carbon hydroelectricity will be used to upgrade the concentrates to spherical coated graphite and other products.
The capital cost to develop the mine and processing facility is estimated to be US$950 million (C$1.24 billion), which includes a contingency of US$130 million (C$170 million).
With a post-tax internal rate of return (8% discount) of 22% and net present value of US$1.04 billion (C$1.36 billion), the financially robust operations are expected to pay back the capital in 5.1 years.
These figures do not take into account the tax credits offered to suppliers of EV battery materials under the Inflation Reductions Act.
Under this legislation, companies that produce lithium-ion battery materials in the U.S. qualify for a tax credit equal to 10% of the production costs. This credit begins to fade by 25% per year starting in 2030.
The Inflation Reduction Act also offers a second tax credit equal to 10% of the costs incurred in respect to the production of 99.9% graphite in the U.S. This credit does not have a sunset date.
The Graphite One projects detailed in the PFS are expected to qualify for both credits.
The company also plans to establish a lithium-ion battery recycling facility alongside its processing plant.
"With this new proposed recycling division joining our Graphite Creek mine and Advanced Graphite Materials Manufacturing Plant as the third link, Graphite One plans to bring the full circular economy to the U.S. graphite supply chain," said Graphite One President and CEO Anthony Huston.
Quebec graphite
Environmentally responsible production of EV anode material lies at the very foundation of Nouveau Monde's strategy to deliver carbon-neutral graphite from the supply chain it is developing in Quebec.
"Battery minerals cannot power a sustainable energy revolution unless their extraction and value-added transformation are done on a 'Zero-Harm' basis," said Nouveau Monde Graphite Chairman Arne Frandsen.
A feasibility study updated in July details plans for a mine at its Matawinie project that is expected to produce an average of 103,328 metric tons of high-purity flake graphite concentrate per year.
This graphite concentrate will be trucked roughly 95 miles (150 kilometers) to the company's advanced material plant at Bécancour, where it will be upgraded to 42,616 metric tons of the coated spherical anode material for lithium batteries and 3,007 metric tons of large flake graphite for other industrial purposes.
"NMG is positioning itself as North America's largest, fully integrated natural graphite production to relieve battery and EV manufacturers from their overreliance on Chinese production," said Nouveau Monde Graphite President and CEO Eric Desaulniers.
As a forward-looking company that is supplying a vital ingredient to the lithium-ion batteries storing renewable energy and powering electric vehicles, Nouveau Monde is shrinking the carbon dioxide footprint of its Quebec operations.
To accomplish this, the company plans to have an all-electric fleet of mining equipment charged with Quebec's abundant hydroelectricity digging up and hauling the graphite at Matawinie.
This idea is so ahead of the curve that the electric mining equipment it plans to use has not been invented yet.
To overcome this hurdle, Nouveau Monde partnered with Caterpillar Inc. to develop, test, and produce a fleet of all-electric Cat mining equipment for its coming graphite mine in Quebec – a landmark collaboration for Nouveau Monde and the mining sector at large.
Nouveau Monde Graphite Inc.
Nouveau Monde's battery material plant in Becancour, Quebec, will upgrade graphite concentrates into battery-grade anode material and flake graphite for other industrial uses.
"We are proud to be a driving force for our peers as we strive to electrify our operations to meet our carbon neutrality commitments while maintaining the productivity and efficiency standards of our mining operations," said Desaulniers. "Even more gratifying and important to our corporate mission is that our project can serve as a springboard for the future of the mining industry by collaborating with Caterpillar on these cutting-edge technologies."
As an added bonus for Nouveau Monde, each of the battery-powered Cat mining machines will need about a ton of graphite.
Desaulniers told Data Mine North that battery manufacturers interested in securing Nouveau Monde Graphite have expressed interest in supplying Caterpillar with the batteries to power its electric machinery at Matawinie and around the globe.
Nouveau Monde plans to begin delivering ESG-boosting graphite into EV supply chains in 2023.
"I am confident that the ESG-minded team at NMG can capitalize on our exclusive ecotechnologies and industry-leading practices to position the company as a Western World's trailblazer for competitive, sustainable, and local graphite advanced materials production," said Frandsen.
Canada aims to speed up new projects with critical minerals strategy
Originally posed on Reuters.com
By Steve Scherer
FILE PHOTO: Canada's Minister of Natural Resources Jonathan Wilkinson speaks during Question Period in the House of Commons on Parliament Hill in Ottawa, Ontario, Canada, April 7, 2022. REUTERS/Patrick Doyle
Canada on Friday unveiled a new strategy to ramp up production and processing of critical minerals vital to power electric vehicle (EV) batteries as the world seeks to shift away from fossil fuels toward cleaner technologies.
The government pledged to review the permitting process with an eye on cutting the time required to bring mines online by avoiding duplication and ensuring early indigenous consultation and engagement, the 58-page strategy document said. It did not say when the review would be completed.
The plan focuses on expanding the critical minerals sector and “moving things forward expeditiously,” Canada’s Natural Resources Minister Jonathan Wilkinson said at a news conference in Vancouver.
Wilkinson said the strategy, backed by nearly C$4 billion ($2.95 billion) allocated in the 2022 budget, “is a roadmap for the creation of wealth and sustainable jobs throughout the value chain in every region of this country.”
Under the strategy, companies will be offered help in applying for permits and for federal support through the Critical Minerals Centre of Excellence. The office is already operating with a team of about 15 people, said a senior government source.
Critical minerals are key elements in EV batteries, electronics and solar panels and play a crucial role in the transition to the green economy. While Canada is home of some the largest deposits of critical minerals, it can take anywhere between five and 25 years for a mining project to become operational, the document said.
“We recognize that, although responsible regulations are vital, complex regulatory and permitting processes can hinder the economic competitiveness of the sector and increase investment risk for proponents,” the document reads.
As the world shifts to cleaner technologies, demand for critical minerals is expected to skyrocket. Many in the mining industry have said the bureaucracy is too slow and is holding up investment.
“What has been the tendency over many years is that the requirements just keep ballooning and you end up with impact assessment reports (for new mines) that are thousands of pages long,” said Pierre Gratton, president and chief executive of the Mining Association of Canada, adding that he welcomed a review of the permitting process.
“We’re not looking for yet another overhaul of the federal regime. We’re looking at opportunities to implement existing requirements more expeditiously. ... There are definitely opportunities to speed things up,” he said.
China dominates the market for critical minerals used in EV batteries.
Countries like the United States, Canada, Australia, India and Japan want to wean themselves from their dependence on authoritarian regimes for strategically important materials.
‘EVERYONE WANTS A PIECE OF CANADA’
Canada signed a joint action plan with the United States in 2020 to advance secure supply chains for critical minerals. It has signed similar critical minerals cooperation agreements with Japan and the European Union.
“I’ve talked to the Americans more in the last few years than I had ever talked to them in my entire career, and also Europeans,” Gratton said. “Everyone wants a piece of Canada right now, and they keep knocking on our door.”
The new strategy lays out priorities along the entire critical-mineral value chain, from exploration and mining to recycling old batteries, and it also covers the need for new infrastructure in often remote corners of the country.
Ottawa will seek “regulatory harmonization” with the United States on critical minerals, the document reads without elaborating.
Ottawa’s strategy prioritizes developing lithium, graphite, nickel, cobalt, copper and rare earth elements. At the same time, Canada is offering a 30% tax credit to spur exploration for nickel, lithium, cobalt, graphite, copper, rare earth elements, vanadium and uranium.
($1 = 1.3579 Canadian dollars)
Canada places big bets on critical minerals
Originally posted on Mining.com
By Nelson Bennett
Canada is blessed with an abundance of fossil fuels — oil, natural gas and coal.
But as the developed world tries to wean itself off of fossil fuels – largely through electrification – it is expected the demand for Canada’s fossil fuels will eventually decline, while demand for critical minerals and metals is projected to grow sixfold by 2040.
These metals and minerals are critical in manufacturing electric vehicle batteries, solar and wind power installations, transmission lines and all the other things that a global energy transition will require.
The Canadian government is hoping to capitalize on the opportunity this poses with a new critical minerals strategy — backed with $4 billion in funding in the recent federal budget — that aims to develop a full critical minerals industry value chain, from exploration and mining, to processing, manufacturing and recycling.
Federal Natural Resources Minister Jonathan Wilkinson is in Vancouver today to release the new strategy.
“By investing in critical minerals today, we are building a sustainable industrial base to support emission-reducing supply chains that will address climate change for generations to come,” the Canadian Critical Minerals Strategy states.
Citing Clean Energy Canada, the strategy estimates $5.7 billion to $24 billion in GDP could be created by 2030 annually by developing a battery supply chain, creating 18,500 to 81,000 direct jobs.
“These figures grow to between $15 billion and $59 billion in annual GDP contributions, and 79,000 and 333,000 jobs, when indirect and induced activities and jobs are included,” the strategy says. “Once realized, these activities would contribute between $2.7 billion and $11 billion annually in combined federal and provincial government revenues.”
Map of mines and development projects in Canada. (Image courtesy of Government of Canada.| Click on map to enlarge it)
Not everyone is convinced Canada has what it takes to become a critical minerals powerhouse, however. Namely, it just doesn’t have the mineral reserves that regions like South America and Africa have, say Philip Bazel and Jack Mintz of the University of Calgary’s School of Public Policy.
In a brief published earlier this week, they suggest Canada will remain a minor player in critical minerals production, simply because it doesn’t have the massive reserves of copper, lithium, cobalt and other critical minerals that countries like Chile and the Democratic Republic of Congo have.
Based on reserves and production of eight critical minerals and metals, among the top six producers, Canada ranks last, according the Bazel-Mintz brief.
They estimate Canada’s global share of copper reserves to be just 1.1% — compared to Chile’s 22.7% — and production at 2.8%. It’s estimated share of nickel and zinc reserves are roughly 2%. Canada’s share of global nickel production was 6.7% in 2020; its share of zinc production was 6%. Canada’s reserves share of cobalt is 2.9% and its production share 2.6%. Its global share of lithium, bauxite and manganese production is currently zero, according to the Bazel-Mitz brief.
“Most of North America’s critical transition minerals will have to come from reserves in South America, Africa, and the Caribbean as well as Australia and China, which will see economic growth from mining jobs and capital investments,” they write.
“Shifting away from carbon-emitting fossil fuels toward cleaner, renewable sources of electric energy will require no less than an order of magnitude more mined minerals and rare earth elements, and Canada has a limited share of these transition minerals.”
But reserve estimates are based on what is known, and there may be more deposits in Canada yet to be discovered. The strategy earmarks $79 million for public geoscience and exploration aimed at discovering potential new deposits. Moreover, Canada’s new critical minerals strategy doesn’t just focus on raw resources. It proposes an end-to-end industry value chain, from exploration and mining, to processing, manufacturing and recycling.
Ottawa is also hoping a Canadian critical minerals industry will be able to piggyback on American policies, like the Inflation Reduction Act, which will pump billions into things like electric vehicles, potentially opening up opportunities for Canada and the U.S. to cooperate on the development North American supply chains.
“Where critical minerals are not used solely for domestic manufacturing, there is value to be captured by increasing exports for allies, and expanding domestic refining, processing and components manufacturing,” the strategy states. “Examples of these minerals are vanadium, gallium, titanium, scandium, magnesium, tellurium, zinc, niobium, and germanium, along with potash, uranium and aluminum.”
Of the 31 critical minerals identified in the strategy, six are “prioritized” – lithium, graphite, nickel, cobalt, copper, and rare earth elements.
Canada already produces some nickel, cobalt and copper – B.C. being the biggest copper producer. And Saskatchewan is a major producer of uranium, which is among the 31 minerals identified in the new strategy.
There are, as yet, no operating lithium mines in Canada, although a proposed new lithium mine in Quebec is now making its way through the Impact Assessment Agency process.
Quebec is home to lithium projects in varied stages of development. (Image courtesy of North American Lithium.)
The exploration and mining sector in Canada may need some prodding to convince it to switch its focus from gold and coal, however. A casual reading of the list of mining projects currently in the Impact Assessment Agency queue underscores that mining in Canada is still focused mainly on gold and metallurgical coal mining.
Of the 26 development projects listed, only five are for minerals and metals other than gold or coal. They include a nickel mine in Ontario, a lithium mine in Quebec, an iron mine in Labrador, a niobium mine in B.C. and also a lead-zinc mine in B.C.
“Although Canada does not possess large quantities of critical minerals relative to global totals, Canadian reserves of cobalt, copper, nickel and zinc represent the best opportunities for growth,” Bazel and Mintz say in their brief. “However, with the majority of these critical mineral reserves abroad, we wonder if Canada’s industry is positioned to compete for the international mining investment.
“Given Canada’s limited share of global energy transition minerals, securing Canadian participation in the energy transition mining market may indeed hinge on the shape of its regulatory and taxation framework for mining companies.”
The strategy does provide some tax incentives, notably a new 30% flow-through tax credit for critical minerals exploration.
Minister Wilkinson Releases Canada’s $3.8-billion Critical Minerals Strategy to Seize Generational Opportunity for Clean, Inclusive Growth
Originally posted on Canada.ca
By Natural Resources Canada
Critical minerals are not just the building blocks of clean technology like solar panels and electric vehicle batteries – they are a key ingredient for creating middle class jobs and growing a strong, globally competitive Canadian economy. The move toward a global net-zero economy is generating a significant increase in demand for critical minerals around the world, creating a generational opportunity for Canadian workers and Canadian businesses. Concurrent geopolitical dynamics have caused like-minded countries to reflect on the need to have stable and secure resources and the clean technologies they enable. There is no global energy transition without accelerated activity in the critical minerals space.
It is in this context that the exploration, extraction, processing, product manufacturing and recycling of critical minerals presents a generational opportunity for Canada. The Government of Canada is committed to seizing this opportunity in a way that creates good jobs and economic opportunity in every region of the country while achieving Canada’s ambitious climate goals and advancing reconciliation, and contributing to global security and supply chain resilience.
Today, in Vancouver, the Honourable Jonathan Wilkinson, Canada’s Minister of Natural Resources, released Canada’s Critical Minerals Strategy, backed by up to $3.8 billion in federal funding allocated in Budget 2022. The proposed funding covers a range of industrial activities, from geoscience and exploration to mineral processing, manufacturing and recycling applications, including support for research, development and technological deployment.
The Strategy maps out how Canada can seize this generational opportunity in a way that accomplishes five key outcomes:
The Strategy focuses on opportunities at every stage along the value chain for Canada’s 31 critical minerals, from exploration to recycling. It is the result of extensive consultations that have validated the Government of Canada’s approach to date — including with respect to the opportunities from lithium, graphite, nickel, cobalt, copper, rare earth elements, potash, uranium and aluminum as outlined in June’s Discussion Paper.
Critically, the Strategy outlines concrete measures to accelerate regulatory processes at the sub-national, national and international levels; to ensure meaningful and ongoing Indigenous partnership throughout the value chain; and to ensure that the Strategy is in line with Canada’s ambitious climate and nature protection goals.
The Strategy builds on work already underway within the government, including historic investments over the past year throughout the critical minerals value chain and a recent approval of a palladium mine in the critical minerals space that delivers on our key desired outcomes.
Today’s announcement is one of a series of significant steps the Government of Canada continues to take to support sustainable jobs and protect the environment. Minister Wilkinson will continue to work with all partners to establish Canada as the global supplier of choice for clean energy in a net-zero world — ensuring a prosperous and clean future for Canadians from coast to coast to coast.
Graphite deficit starting this year, as demand for EV battery anode ingredient exceeds supply
Originally posted on Mining.com
By Richard Mills
Flake graphite. (Image by 2×910, Wikimedia Commons).
Although EV market share is still tiny compared to traditional vehicles, that is likely to change in the coming years as major economies transition away from fossil fuels and move into clean energy.
US President Joe Biden has signed an executive order requiring that half of all new vehicle sales be electric by 2030. China, the world’s biggest EV market, has a similar mandate that requires electric cars to make up 40% of all sales. The European Union is also seeking to have at least 30 million zero-emission vehicles on its roads by then.
According to the IEA’s Global Electric Vehicle Outlook, if governments are able to ramp up their efforts to meet energy and climate goals, the global electric vehicle fleet could reach as high as 230 million by the end of the decade, compared to about 20 million currently.
With more electric cars comes the need for more raw materials like lithium, nickel and graphite to build batteries. The IEA believes mineral demand for use in EVs and battery storage must grow at least 30 times by 2040 to meet various climate goals.
Fastmarkets forecast that EV sales will experience a compound annual growth rate of 40% per year through 2025, when EV penetration is expected to reach 15%. After that, EV market share is expected to rise further, reaching 35% by 2030.
One mineral that has been overlooked, but is an essential part of vehicle electrification, is graphite.
At AOTH, we believe graphite represents a “backdoor” market opportunity brought about by the clean energy transition. This is for several reasons:
Graphite as anode material
The lithium-ion battery used to power electric vehicles is made of two electrodes — an anode (negative) on one side and a cathode (positive) on the other. At the moment, graphite is the only material that can be used in the anode, there are no substitutes.
This is due to the fact that, with high natural strength and stiffness, graphite is an excellent conductor of heat and electricity. Being the only other natural form of carbon besides diamonds, it is also stable over a wide range of temperatures.
The cathode is where metals like lithium, nickel, manganese and cobalt are used. Depending on the battery chemistry, there are different options available to battery makers (see below).
Graphite is thus considered indispensable to the global shift towards electric vehicles. It is also the largest component in lithium-ion batteries by weight, with each battery containing 20-30% graphite. But due to losses in the manufacturing process, it actually takes 30 times more graphite than lithium to make the batteries.
According to the World Bank, graphite accounts for nearly 53.8% of the mineral demand in batteries, the most of any. Lithium, despite being a staple across all batteries, accounts for only 4% of demand.
An electric car contains more than 200 pounds (>90 kg) of coated spherical purified graphite (CSPG), meaning it takes 10 to 15 times more graphite than lithium to make a Li-ion battery.
Graphite is so essential to a lithium battery, that Tesla’s Elon Musk famously said, “Our cells should be called Nickel-Graphite, because primarily the cathode is nickel and the anode side is graphite with silicon oxide.”
Demand overflow
The anode material, called spherical graphite, is manufactured from either flake graphite concentrates produced from graphite mines, or from synthetic/artificial graphite. Only flake graphite upgraded to 99.95% purity can be used.
An average plug-in EV has 70 kg of graphite, or 10 kg for a hybrid. Every 1 million EVs requires about 75,000 tonnes of natural graphite, equivalent to a 10% increase in flake graphite demand.
According to Benchmark Mineral Intelligence (BMI), the flake graphite feedstock required to supply the world’s lithium-ion anode market is projected to reach 1.25 million tonnes per annum by 2025. The amount of mined graphite for all uses in 2021, was just 1 million tonnes. (USGS)
Furthermore, the London-based price reporting agency forecasts demand for graphite from the battery anode segment could increase by seven times in the next decade as the growth in EV sales continues to drive construction of lithium-ion megafactories.
BloombergNEF expects demand for battery minerals to remain robust through 2030, with graphite demand increasing four-fold.
The International Energy Agency (IEA) goes 10 years further out, predicting that growth in demand for selected minerals from clean energy technologies by scenario, 2020 relative to 2040, will see: increases of lithium 13x to 42x, graphite 8x to 25x, cobalt 6x to 21x, nickel 7x to 19x, manganese 3x to 8x, rare earths 3x to 7x, and copper 2x to 3x.
Supply squeeze
As vehicle electrification continues, and few new sources are discovered worldwide, BMI estimates the graphite market could reach a deficit as early as this year, with the supply shortfall growing to 8Mt by 2040; to fill this gap, the mining industry would need to produce nearly 8x as much graphite as it does currently, over the next 18 years.
On June 7, in an article titled ‘How a battery metals squeeze puts EV future at risk’, The Washington Post reported, Factory lines churning out power packs to fuel a clean energy future are being built faster than strained supply chains can keep up. A global rush to lock in stocks of lithium, nickel, cobalt and other key ingredients from a handful of nations has sent prices hurtling higher… While factories can be built in about 18 months, mines can typically take seven years or longer to come online.
June saw repeated concerns over the supply of battery metals forecast for the decade ahead, including from Tesla. CEO Elon Musk reportedly explained that production has been hindered by raw material shortages and shutdowns of assembly lines in China.
Lack of diverse supply
Almost all graphite processing today takes place in China because of the ready availability of graphite there, weak environmental standards and low costs. Nearly 60% of the world’s mined production last year also came from China, making it a dominant player in every stage of the graphite supply chain.
After China, the next leading graphite producers are Mozambique, Brazil, Madagascar, Canada and India. The US does not produce any natural graphite, therefore it must rely solely on imports to satisfy domestic demand.
The level of foreign dependence has increased over the years. The US imported 38,900 tonnes of graphite in 2016, then peaking at 70,700 tonnes in 2018.
The latest publication from the USGS shows that imports in 2021 totaled 53,000 tonnes, of which 71% was high-purity flake graphite, 42% was amorphous, and 1% was lump and chip graphite.
The main import sources were China (33%), Mexico (21%), Canada (17%) and India (9%).
Since China controls all spherical graphite processing, the US is not actually 33% dependent on China for its battery-grade graphite, but 100%.
This is why the US government has included graphite among the 35 minerals that it deems “critical to its national security and economy.”
A White House report on critical supply chains showed that graphite demand for clean energy applications will require 25 times more graphite by 2040 than was produced worldwide in 2020.
Graphite pricing
The value of natural graphite has increased significantly over the course of the past year, with demand continuing to outstrip supply. According to Benchmark Mineral Intelligence, prices have gone up steadily since January 2021 on all types of graphite, with fines increasing 44.50% from USD$500/ton in January of 2021 to $723/t in May of 2022. Using those same dates, large flake graphite prices climbed 19.85% from $983/t to $1,187/t, and spherical graphite rose 8.39% from $2,958/t to $3,207/t.
More recently, flake and spherical graphite prices are both up slightly. According to Fastmarkets, for the week ending June 16, the spot price of China flake graphite 194 EXW was up 0.37% over 30 days, and 19.39% over 360 days. Graphite produced at 94-97% purity is considered best suited for batteries, before it is upgraded to 99.99% purity to make spherical graphite. Spherical graphite 99.95% min EXW China was up 1.58% over the past 30 days, for the week ended June 16.
China flake graphite 94% C (-100 mesh) was priced at $830 per ton, with Europe flake graphite of the same grade and size selling for $920/t.
Conclusion
During a time of price weakness for a number of industrial metals (copper, zinc, aluminum, for example), the price of graphite, being critical to the electric-vehicle transition, has held up extremely well.
Flake and spherical graphite are both trending higher, in fact the prices of all types of graphite (fines, large flake, spherical) have increased significantly since January 2021, on the back of robust demand from battery-makers and EV manufacturers, and limited supply.
According to BMI, in 2022 demand for lithium-ion batteries is growing at its fastest ever, on course for a year-on-year growth rate of nearly 50%.
While this will increase the need for other battery minerals, such as lithium, nickel and cobalt, graphite remains the highest-intensity mineral in the lithium-ion battery by weight, with over 570,000 tonnes of natural flake to be consumed in 2022.
Yet as Seeking Alpha observes, consumer demand for electric vehicles surpasses our ability to supply them. Waiting times for EVs are lengthening, a lithium ion battery shortage is hitting many automakers, and, most crucially, key raw material prices are at all-time highs.
This bodes well for companies with large graphite deposits in safe jurisdictions, that can not only capitalize on high prices, but contribute to the local graphite supply chain and lessen the dependence on China for graphite mining and especially, graphite processing.
For years neglected by governments, critical minerals like graphite are finally getting the attention they deserve. In June, the Canadian government unveiled its low-carbon industrial strategy, that will see Ottawa partnering with each province to “identify, prioritize and pursue opportunities”. Specific to critical minerals, this means battery manufacturing in Quebec and electric vehicle production in Ontario.
Natural Resources Minister Jonathan Wilkinson pointed to CAD$3.8 billion already earmarked for critical minerals in the April budget. On top of that, “we have a billion and a half dollars in the Clean Fuels Fund, we have eight billion dollars in the Net Zero Accelerator, we’re setting up the Clean Growth Fund, we have the Canada Infrastructure Bank,” Bloomberg quoted him saying. He added:
“The average mine takes 15 years to bring into production. In the context of the energy transition, we don’t have 15 years if we’re actually going to provide enough of the minerals to be able to support just the battery development. So it behooves us to bring everybody into the room to figure out how to do it.”
At AOTH, we couldn’t agree more. Canada’s new industrial strategy dovetails with what is happening south of the border.
The US, which has long sought to improve its battery supply chain, recently invoked its Cold War powers by including lithium, nickel, cobalt, graphite and manganese on the list of items covered by the 1950 Defense Production Act, previously used by President Harry Truman to make steel for the Korean War.
To bolster domestic production of these minerals, US miners can now access $750 million under the act’s Title III fund, which can be used for current operations, productivity and safety upgrades, and feasibility studies. The DPA could also cover the recycling of these materials.
Later this year, the Department of Energy will begin doling out $6 billion in grants for battery production, half of which are earmarked for domestic supplies of materials and battery recycling.
The Biden administration has already allocated $6 billion as part of the $1.2 trillion infrastructure bill, towards developing a reliable battery supply chain and weaning the auto industry off its reliance on China, the biggest EV market and leading producer of lithium-ion cells.
Among the minerals key to winning the global EV race, graphite arguably is most significant and should be a top priority for the US, given it is the essential ingredient in electric vehicle batteries.
A global graphite shortage is a matter of when, not if, without new sources of supply. For the US, which is 100% dependent on foreign imports of the material, it’s a ticking time bomb that could completely derail the nation’s vehicle electrification and decarbonization ambitions.
This all goes back to the importance of establishing a reliable, secure and sustainable “mine to battery” EV supply chain, beginning with a domestic graphite source and integrating it with processing, manufacturing and recycling to create a full circular economy.
Graphite Miners News For The Month Of November 2022
Originally posted on Seekingalpha.com
By Trend Investing
Graphite price news
During the past 30 days the China graphite flake-194 EXW spot price was up 0.22%. The China graphite flake-199 EXW spot price was up by 0.16%. Note that 94-97% is considered best suited for use in batteries; it is then upgraded to 99.9% purity to make "spherical" graphite used in Li-ion batteries. The spherical graphite 99.95% min EXW China price was up 0.28% the past 30 days.
Fastmarkets (see below, not updated) shows China graphite flake 94% C (-100 mesh) prices at US$830/t and Europe graphite flake 94% C (-100 mesh) prices at US$920/t. Note: Fastmarkets stated recently: "The most recent price assessments for graphite flake, 94% C, -100 mesh, cif Europe, and graphite flake, 94% C, -100 mesh, fob China, were $800 and $810 per tonne respectively on September 22."
Fastmarkets graphite prices the week ending June 16, 2022 (not updated)
2021 IEA forecast growth in demand for selected minerals from clean energy technologies by scenario, 2040 relative to 2020 - Increases Of Lithium 13x to 42x, Graphite 8x to 25x, Cobalt 6x to 21x, Nickel 7x to 19x, Manganese 3x to 8x, Rare Earths 3x to 7x, And Copper 2x to 3x
2022 - BMI forecasts graphite deficits to begin from 2022 as demand for graphite grows strongly
2022 - BMI forecasts we need 330+ new EV metal mines from 2022 to 2035 to meet surging demand - 97 new 56,000tpa natural flake graphite mines
Graphite market news
On November 1 The Business Times reported:
South Korea launches government-backed battery alliance to source key metals...... The country, home to major battery makers LG Energy Solution, Samsung SDI, and SK Innovation's SK On, is seeking to bolster supply chain stability and metals to become a major player in the field, which is dominated by China.......
On November 8 Bloomberg reported:
Democrats supercharged EV investment while they had the chance...... More than $13 billion of investment in battery raw material production and battery and EV manufacturing has been announced in the less than three months since Biden signed the IRA into law on Aug. 16. Volkswagen and Mercedes-Benz almost immediately sealed agreements to secure mining and refining resources from America's neighbor to the north. Honda and Toyota earmarked almost $7 billion worth of EV battery plant investments within two days of one another. An Australian development company started up the first US cobalt mine in three decades. BMW said it would spend $1.7 billion expanding its South Carolina SUV factory, and that its battery supplier would build a new plant nearby.
Democrats supercharged EV investment. Battery manufacturing being the main winner so far (Source: Bloomberg)
On November 11 BloombergBNN reported:
Ford, GM in talks with Posco on investing in battery metal hubs. Ford Motor Co., General Motors Co., and Stellantis NV are in talks with South Korea's Posco Chemical Co. about potentially investing in plants producing electric-vehicle battery materials in North America, according to people familiar with the matter. The factories would make cathode-active or anode materials......
On November 13 CBS News reported:
U.S. military weighs funding mining projects in Canada amid rivalry with China. Canadian companies told they qualify under Defense Production Act...... The United States military has been quietly soliciting applications for Canadian mining projects that want American public funding through a major national security initiative.
On November 23 Mining.com posted the chart below. It shows the Benchmark Mineral Intelligence ("BMI") flake graphite balance of demand v supply forecast. For graphite BMI is forecasting the flake graphite market to be balanced 2023 to 2027, then moving into a growing deficit from 2027 to 2035.
graphite-market-balance benchmarkweek-2022 (source)
Graphite miners news
Graphite producers
I have not covered the following graphite producers as they are not typically accessible to most Western investors. They include - Aoyu Graphite Group, BTR New Energy Materials, Qingdao Black Dragon, National de Grafite, Shanshan Technology, and LuiMao Graphite.
Note: AMG Advanced Metallurgical Group NV [AMS:AMG] [GR:ADG] (OTCPK:AMVMF) is also a "diversified producer", producing graphite, vanadium, and lithium. SGL Carbon (ETR:SGL) is a synthetic graphite producer and Novonix [ASX:NVX] (OTCQX:NVNXF) is commercializing their synthetic graphite product. Graphex Group Limited [HK:6128] (OTCQX:GRFXY) makes spherical graphite.
Syrah Resources Limited [ASX:SYR][GR:3S7]( OTCPK:SYAAF)(OTC:SRHYY)
Syrah Resources Limited owns the Balama graphite mine in Mozambique. Syrah is also working to become a vertically integrated producer of natural graphite Active Anode Material ("AAM") at their Vidalia facility, Louisiana, USA.
On October 27, Syrah Resources Limited announced:
Balama update.....Mining and processing operations, and unrestricted logistics movements have resumed at Balama with the full complement of the Company's employees and contractors onsite.....
On November 14 Syrah Resources announced:
Balama update......security concerns in Namuno district around 50 kms from the Balama Graphite Operation.....Syrah has assessed that the safe return of the workforce to site and resumption of operations at Balama will be undertaken from today.
You can view the latest investor presentation here.
Catalysts:
September 2023 quarter - First Stage 11.25ktpa AAM Vidalia facility targeted to start production.
Ceylon Graphite [TSXV:CYL] [GR:CCY] (OTC:CYLYF)
Ceylon Graphite has 'Vein graphite' production out of one mine in Sri Lanka with 121 square kilometers of tenements.
No news for the month.
Mineral Commodities Ltd. ("MRC") [ASX:MRC]
Skaland Graphite is 90% owned by MRC. Skaland is the highest grade flake graphite operation in the world and largest producing mine in Europe; with immediate European graphite production of up to 10,000 tonnes per annum with regulatory approval to increase to 16,000. MRC plans to demerge its Norwegian graphite assets into a newly incorporated Norway company branded as Ascent Graphite.
On October 31, Mineral Commodities Ltd. announced: "Quarterly activities report-September 2022." Highlights include:
During the quarter:
"MRC secures finished product garnet offtake and Mineral Separation Plant funding term sheet with GMA.
MRC granted De Punt prospecting right at South Tormin,2 with subsequent exploration work identifying the high prospectivity of De Punt, which appears to extend the Tormin Western and Eastern Strandline deposits to the south.
Government confirms Critical Minerals Grant funding for MRC to build pilot scale battery anode plant in Australia.
MRC and Green Graphite Technologies sign an Equity/Licence Agreement5 to build alternate process pilot scale battery anode plant in Canada.
Tormin mining and processing throughput remains above budget expectations.
Fourth consecutive quarter of stabilised operating performance at Skaland."
After quarter end.
"MRC Secured Funding Support for its Strategic Plan Growth Strategy."
Corporate and Cash
"Cash: US$1.9 million as at 30 September 2022, with an A$15.7 million Placement and Rights Issue announced for the December 2022 quarter.
Debt: US$9.3 million as at 30 September 2022.
Securities: 558.8 million shares and 27.7 million performance rights as at the date of this report."
Tirupati Graphite [LSE:TGR]
No news for the month.
Northern Graphite [TSXV:NGC][GR:ONG] (OTCQX:NGPHF)
Northern Graphite purchased from Imerys the Lac des Iles producing graphite mine in Quebec and the Okanjande graphite deposit/Okorusu processing plant in Namibia. They also own the Bissett Creek graphite project located 100km east of North Bay, Ontario, Canada and close to major roads and infrastructure. The Company has completed an NI 43-101 Bankable final Feasibility Study and received its major environmental permit.
On November 9, Northern Graphite announced:
Northern Graphite third quarter operational and development project update. The LDI operation continued to perform well in the third quarter with 53,263 tonnes of ore milled at an average feed grade of 6.4% graphite........
You can view the latest investor presentation here and the latest Trend Investing article on Northern Graphite here or the very recent and excellent Trend Investing CEO interview here.
Graphite developers
NextSource Materials Inc. [TSX:NEXT] [GR:1JW] (OTCQB:NSRCF)
NextSource Materials Inc. is a mine development company based in Toronto, Canada, that's developing its 100%-owned, Feasibility-Stage Molo Graphite Project in Madagascar. The Company also has the Green Giant Vanadium Project on the same property. The Molo mine is fully-funded and scheduled to commission in December, 2022.
No news for the month.
Investors can view the latest company presentation here or the latest Trend Investing article here.
Talga Group [ASX:TLG] [GR:TGX] (OTCPK:TLGRF)
Talga Group is a technology minerals company enabling stronger, lighter and more functional materials for the multi-billion dollar global coatings, battery, construction and carbon composites markets using graphene and graphite. Talga 100% owned graphite deposits are in Sweden, proprietary process test facility is in Germany.
On October 31, Talga Group announced: "Quarterly activities review for period ending 30 September 2022." Highlights include:
Commercial and project development
"Automotive Cells Company ("ACC") signs non-binding offtake for 60,000 tonne anode supply....."
Product and technology development
"Talga receives funding to further Talnode®-Si commercialisation....."
Corporate and finance
"Oversubscribed A$22 million institutional placement completed subsequent to quarter.
Oversubscribed A$10 million Share Purchase Plan completed subsequent to quarter.
Talga and Mitsui extend Vittangi Anode Project MoU......"
On November 3, Talga Group announced:
Vittangi environmental permit hearing date. The hearing is scheduled to take place in Luleå, commencing the week of 30 January2023 and expected to conclude the week of 20 February 2023.....
On November 23, Talga Group announced:
Advanced progress with European Investment Bank for Vittangi Anode Project funding..... The Project will use 100% renewable electricity to extract graphite, an EU defined critical material, and refine it into coated anode for Li-ion batteries. The first stage of the Project will produce 19,500tpa of anode for 24 years from the integrated mine-to-anode operation (ASX:TLG 1 July 2021). EIB's potential financing commitment of up to EUR300m, pending final due diligence, credit approvals and agreements, is foreseen to cornerstone and complement debt funding discussions underway with a consortium of leading export credit agencies and international banks.
You can view the latest investor presentation here.
Westwater Resources (NYSE:WWR)
Westwater Resources Inc. is developing an advanced battery graphite business in Alabama. The Coosa Graphite Plant (2023 production start) plans to source natural graphite initially from non-China suppliers and then from the USA from 2028.
On November 10, Westwater Resources Inc. announced: "Westwater Resources, Inc. announces results for third quarter ended September 30, 2022." Highlights include:
".....Net cash used in investing activities of $32.0 million for the nine months ended September 30, 2022, relates to construction spend for Phase I of the Kellyton graphite plant......
Consolidated net loss for the third quarter of 2022 was $3.5 million, or $0.07 per share....
Cash and working capital as of September 30, 2022, were $100.3 million and $80.1 million...."
You can view the latest investor presentation here.
Magnis Energy Technologies Ltd [ASX:MNS] (OTCQX:MNSEF)
Magnis is an Australian based company that has rapidly moved into battery technology and is planning to become one of the world's largest manufacturers of lithium-ion battery cells. Magnis has a world class graphite deposit in Tanzania known as the Nachu Graphite Project.
On October 31, Magnis Energy Technologies Ltd. announced: "Quarterly report for quarter ending September 2022." Highlights include:
"Magnis' Lithium-ion battery manufacturing facility operated by Imperium3 New York Inc ("iM3NY") commences commercial production and begins scale up phase towards Gigawatt scale. At capacity, iM3NY expects to produce ~15,000 cells per day.
Bankable Feasibility Study Update confirms strong financial and technical viability for the Nachu Graphite Project.
Positive results continue in C4V's Extra Fast Charging battery program using 7Ah (Amp hour) commercial graded cells with 20 minute-charge and 20-minute discharge. Results show only 3% loss of the initial cell capacity after approximately 2600 cycles.
Magnis' US traded OTC shares (OTC: MNSEF) has been approved by the US' Depository Trust Company for real-time electronic trading and settlement in USD making it easier and cheaper for US investors."
Gratomic Inc. [TSXV:GRAT] [GR:CB82 ] (OTCQX:CBULF)
Gratomic's Aukam Graphite Project is located in Namibia, Africa. The Project is undergoing 'operational readiness'. Gratomic also 100% own the Capim Grosso Graphite Project in Brazil. Gratomic is also collaborating with Forge Nano to develop a second facility for graphite micronization and spheronization.
On November 15, Gratomic Inc. announced: "Gratomic announces completion of NI43-101 Technical Report for 100% owned Capim Grosso Project.....in Brazil "
On November 17, Gratomic Inc. announced: "Gratomic announces assay results on Aukam Diamond Drilling Program." See the news release for details. Drill lengths were very short.
Black Rock Mining [ASX:BKT]
On October 28, Black Rock Mining announced: "Quarterly activities/appendix 5B cash flow report." Highlights include:
"Front End Engineering Design process completed, reconfirming Mahenge as a significant Tier 1 scale project with attractive forecast returns.
Conditional Framework Agreement signed with US cleantech graphite processing company, Urbix, Inc.
Special Mining Licence awarded for Mahenge.
Resettlement activities commenced covering areas for planned Modules 1 and 2.
Initial Tanzanian leadership appointments made with first Board constituted under joint-venture Company, Faru Graphite Corporation.
Debt financing process advanced.
Project development and execution activities ongoing.
Graphite market outlook continues to show positive signals with substantial supply deficits predicted in the near-term.
A$20.5M cash at bank as at 30 September 2022."
Nouveau Monde Graphite [TSXV:NOU] (OTCQX:NMGRF) (NYSE:NMG) and Mason Graphite [TSXV:LLG] [GR:M01] (OTCQX:MGPHF)
Nouveau Monde Graphite ("NMG") own the Matawinie graphite project, located in the municipality of Saint-Michel-des-Saints, approximately 150 km north of Montreal, Canada. NMG (51%) and Mason Graphite (49%) have agreed to JV (subject to approvals) on the Lac Guéret Project.
On October 25, Nouveau Monde Graphite announced: "Investor Briefing: Offtake and strategic partnership with Panasonic Energy and Mitsui."
On November 8, Nouveau Monde Graphite announced:
NMG announces the closing of US$50 million Private Placement by Mitsui, Pallinghurst and Investissement Québec..... Through the Private Placement, Mitsui subscribed for US$25 million in Convertible Note, while Pallinghurst and IQ each subscribed for US$12.5 million. The Company intends to use the proceeds of the Private Placement to work in the upcoming months on optimizing the feasibility study dated July 6, 2022, on NMG's Phase-2 Commercial integrated operations, which was filed on SEDAR and EDGAR on August 10, 2022.
On November 11, Nouveau Monde Graphite announced:
NMG reports on quarter progress on the heels of commercial partnership with Panasonic Energy and commencement of expansion planning, and appoints Stephanie Anderson to its Board of Directors.......Period-end cash position of CA $14M and CA $81.5 million on a pro-forma basis.
You can view the latest investor presentation here.
Greenwing Resources Limited [ASX:GW1] (OTCPK:BSSMF) (formerly Bass Metals [ASX:BSM])
On October 31, Greenwing Resources Limited announced: "Quarterly activities report - September 2022 quarter 31 October 2022." Highlights include:
Corporate
"A$12m strategic funding transaction with NIO Inc. announced on 26 September 2022."
Graphmada Graphite Mining Complex, Madagascar
"Updated Graphmada Mineral Resource announced, with 61.9 million tonnes (Mt) at 4.5% Fixed Carbon (FC), nearly tripling the total contained graphite to 2.7 Mt."
You can view the latest company presentation here.
Triton Minerals [ASX:TON][GR:1TG]
Triton Minerals Ltd. engages in the acquisition, exploration and development of areas that are highly prospective for gold, graphite and other minerals. The company was founded on March 28, 2006 and is headquartered in West Perth, Australia. Triton has two large graphite projects in Mozambique, not far from Syrah Resources Balama project.
On October 31, Triton Minerals announced: "Quarterly activities report for period ending 30 September 2022." Highlights include:
"$8.5M Two Tranche Capital Raising - to accelerate development of Ancuabe, including Cornerstone investment of A$5M by major Chinese listed commodities trading and resources company, Shandong Yulong Gold (subject to Shareholder approval and Australian and Chinese regulatory approvals).
A$3.5M Tranche 1 - with institutional and sophisticated investors settled during the quarter.
Cash on hand - as at 30 September 2022 - A$4.8M, with commitments for a further A$5M from Tranche Two of Capital Raising."
You can view the latest investor presentation here and the latest article on Trend Investing here.
Eagle Graphite [TSXV:EGA] (OTCPK:APMFF)
The Black Crystal Project is located in the Slocan Valley area of British Columbia, Canada, 35km West of the city of Nelson, and 70km North of the border to the USA. The quarry and plant areas are the project's two main centers of activity.
No news for the month.
SRG Mining Inc. [TSXV:SRG] [GR:18Y] [Formerly SRG Graphite Inc.]
SRG is focused on developing the Lola graphite deposit, which is located in the Republic of Guinea, West Africa. The Lola Graphite occurrence has a prospective surface outline of 3.22 km2 of continuous graphitic gneiss, one of the largest graphitic surface areas in the world. SRG owns 100% of the Lola Graphite Project.
No news for the month.
You can view the latest investor presentation here.
Leading Edge Materials [TSXV:LEM] (OTCQB:LEMIF)
Leading Edge Materials Corp. is a Canadian company focused on becoming a sustainable supplier of a range of critical materials. Leading Edge Materials' flagship asset is the Woxna Graphite Project and processing plant in central Sweden. The company also owns the Norra Karr REE project, and the 51% of the Bihor Sud Nickel-Cobalt exploration stage project in Romania.
No significant news for the month.
Investors can view the latest company presentation here.
Renascor Resources [ASX:RNU](OTC:RSNUF)
Renascor Resources Ltd. is an Australian exploration company, which focuses on the discovery and development of economically viable deposits containing uranium, gold, copper, and associated minerals. Its projects include graphite, copper, precious metals, and uranium.
On October 31, Renascor Resources announced:
Quarterly activities report for the period ended 30 September 2022.... Renascor secured a site for its proposed state-of-the-art Battery Anode Material (BAM) Manufacturing Facility from South Australian Government-owned utility SA Water.....Siviour is currently the second largest reported Proven Graphite Reserve in the world and the largest Graphite Reserve outside Africa2, supporting a 40-year mine life with production of Graphite Concentrates up to 150,000 tonne per annum3......Renascor is progressing work on an updated, optimised BAM study (BAM Study) that is assessing an increase in Stage 1 PSG production capacity, as well as additional staged expansions of PSG operations in order to meet projected demand. Studies to date have considered an initial Stage 1 production capacity of 28,000tpa PSG4. Renascor's cash position as of 30 September 2022 was approximately $71 million.
You can view the latest investor presentation here.
EcoGraf Limited [ASX:EGR] [FSE:FMK] (ECGFF)
On October 31, EcoGraf Limited announced:
Quarterly activities report. Clean energy initiatives drive strong North American and European Battery Minerals demand.....ISO compliant Life Cycle Assessment demonstrates potential for EcoGraf HFree™ anode material to reduce CO2emissions by over 92% compared to synthetic graphite.....Cash and deposits at end of quarter of $43.4m.
You can view the latest investor presentation here.
Lomiko Metals Inc. [TSXV:LMR] (OTCQB:LMRMF)
Lomiko has two projects in Canada - La Loutre graphite Project (flagship) (100% interest) and the Bourier lithium Project (70% earn in interest).
No news for the month.
Focus Graphite [TSXV:FMS][GR:FKC] (OTCQB:FCSMF)
No news for the month.
Metals Australia [ASX:MLS]
On October 31, Metals Australia announced: "Quarterly activities report for the quarter ended 30 September 2022." Highlights include:
Lac Rainy Graphite Project, Quebec, Canada:
"Final stage of Phase 2 metallurgical testwork at Lac Rainy Graphite Project produced6.5kg high-grade bulk-concentrate (actual LOI grade 95% graphitic carbon, Cg)which was despatched to Germany for spherical graphite and battery testwork.
Initial results of high-purity spherical graphite testwork are expected shortly. The next stage of electrochemical (battery) testwork will follow and this work will determine lithium-ion battery anode charging qualities and durability.
Following the battery testwork the Company plans to testtargets for high-grade graphite resource growth and generate further graphite concentrate for downstream testwork with potential offtakers."
You can view an August 2022 Metals Australia update video here.
Sovereign Metals [ASX:SVM] [GR:SVM][LSE:SVML]
On October 26, Sovereign Metals announced:
Further aircore drilling confirms significant pit expansion potential at depth. This newly defined high-grade rutile and graphite mineralisation at depths >15m is consistent and occurs in coherent blocks.....
On October 31, Sovereign Metals announced: "September 2022 quarterly report."
You can view the latest investor presentation here.
Sarytogan Graphite [ASX:SGA]
Sarytogan Graphite has a 209mt @ 28.5% TGC Inferred Resource (60mt contained graphite) in Central Kazakhstan.
On October 31, Sarytogan Graphite announced: "Quarterly activities report quarter ending 30 September 2022." Highlights include:
"29 diamond drill holes for 2,222m completed to end September at the Sarytogan Graphite Project since April.
Thick high-grade graphite mineralisation reported, including in areas outside of the existing 209Mt @ 28.5% TGC Inferred Mineral Resource...
Drilling is on track to be completed by November 2022 to enable an updated Mineral Resource Estimate in Q1 2023.
Interim step of 92.1% purity graphite concentrate produced by our Australian lab partners using flotation, low-temperature caustic roasting, leaching with a weak sulphuric acid and a final calcine step (Refer ASX Announcement 12/10/22).
Preferred product strategy identified as battery anode material at an optimal micro-crystalline sizing which attracts a premium product price.
Optimisation and purification test work continuing in Germany and Australia."
On November 8, Sarytogan Graphite announced: "High-grade drill results from the central graphite zone." Highlights include:
"Thick high-grade graphite intercepts returned in fourteen diamond drill holes from the Central Graphite Zone (CGZ)......Significant intercepts above 30% TGC or thicker than 60m include: 22.9m @ 30.1% TGC from 16.2m......"
BlackEarth Minerals [ASX:BEM]
On November 3, BlackEarth Minerals announced: "DFS forecasts strong returns for Maniry Project. Study finds Maniry will be financially robust, producing graphite which meets the criteria needed for processing into lithium battery component." Highlights include:
"Maniry Definitive Feasibility Study [DFS]) confirms the Project will generate compelling financial returns and be technically robust. The findings are based on a flowsheet designed by Independent Technical Experts.
The DFS confirms Madagascar is an optimal location for graphite processing and has potential to be the largest producer of graphite outside China.
Tests confirm Maniry graphite will meet the requirements for many value-added products, including lithium batteries and other decarbonisation-related products.
Detailed Environmental & Social Impact Study (ESIA) program is ongoing with the aim of further enhancing Maniry's viability."
DFS highlights for the Maniry Project
BlackEarth Minerals
Zentek Ltd. [TSXV:ZEN] (ZTEK)(formerly ZEN Graphene Solutions Ltd.)
On October 28, Zentek Ltd. announced:
Zentek provides update on Battery Technology Development. "The Canadian government and the auto industry have committed to invest over $10 billion in battery manufacturing in 2022 alone. With the significant push toward electrification in North America, we are excited to be working with the team from U of T, who have worked with a number of international battery manufacturing companies," said Greg Fenton, CEO of Zentek. "This is an important research project to seek to develop next generation graphene-based battery materials to potentially enhance energy density, increase charging rates and improve battery safety."
Other graphite juniors
Armadale Capital [AIM:ACP], BlackEarth Minerals [ASX:BEM], DNI Metals [CSE:DNI] (OTC:DMNKF), Eagle Graphite [TSXV:EGA] [GR:NJGP] (OTC:APMFF), Electric Royalties [TSXV:ELEC], Graphite One Resources Inc. [TSXV:GPH] [GR:2JC] (OTCQX:GPHOF), Green Battery Minerals Inc. [TSXV:GEM] (OTCQB:GBMIF), International Graphite [ASX:IG6], New Energy Metals Corp. [ASX:NXE], Volt Resources [ASX:VRC] [GR:R8L], South Star Battery Metals [TSXV:STS] (OTCQB:STSBF), Walkabout Resources Ltd [ASX:WKT].
Synthetic Graphite companies
SGL Carbon (ETR:SGL)
Novonix Ltd [ASX:NVX](OTCQX:NVNXF)
Graphene companies
Archer Materials [ASX:AXE]
Black Swan Graphene Inc. [TSXV:SWAN]
Elcora Advanced Materials Corp. [TSXV:ERA](OTCPK:ECORF)
First Graphene [ASX:FGR] (OTCQB:FGPHF)
Graphene Manufacturing Group Ltd [TSXV:GMG]
NanoXplore Inc. [TSXV:GRA] (OTCQX:NNXPF)
Strategic Elements Ltd [ASX:SOR]
Zentek Ltd. [TSXV:ZEN] (ZTEK)
Conclusion
November saw only slightly higher flake and spherical graphite prices.
Highlights for the month were:
Democrats supercharged USA EV investment with US$13b of investments announced so far, led by battery manufacturing.
Ford, GM in talks with Posco on investing in battery metal hubs....including anode materials.
U.S. military weighs funding mining projects in Canada amid rivalry with China.
BMI flake graphite forecast is for a balanced market from 2023-2027, then growing deficits from 2027-2035.
Syrah Resources - Balama operations resume.
Mineral Commodities - Government confirms Critical Minerals Grant funding for MRC to build pilot scale battery anode plant in Australia.
Talga Group - Automotive Cells Company signs non-binding offtake for 60,000 tonne anode supply. Advanced progress with European Investment Bank for Vittangi Anode Project funding.
Nouveau Monde Graphite signs MoU with Panasonic Energy to confirm intentions for a multi-year offtake agreement for a significant portion of NMG's active anode material. US$50 million Private Placement by Mitsui, Pallinghurst and Investissement Québec.
Greenwing Resources A$12m strategic funding transaction with NIO Inc.
Metals Australia Final stage of Phase 2 metallurgical testwork at Lac Rainy Graphite Project produced6.5kg high-grade bulk-concentrate.
Sovereign Metals further aircore drilling confirms significant pit expansion potential at depth.
BlackEarth Minerals Maniry DFS confirms the Project will generate compelling financial returns and be technically robust.
Goldman Sachs says US, Europe could end reliance on Chinese EV batteries by 2030
Originally posted on Mining.com
By Jahnavi Nidumolu; Editing by Tom Hogue and Jamie Freed
Battery packs are assembled. (Image: Stefan Warter AUDI AG)
The United States and Europe could cut their dependence on China for electric vehicle batteries through more than $160 billion of new capital spending by 2030, the Financial Times reported on Monday, citing a Goldman Sachs forecast.
The investment bank’s analysts believe demand for finished batteries could be met without China within the next three to five years, as a result of investments in the US by South Korean conglomerates LG and SK Hynix, according to a Goldman report to clients viewed by the newspaper.
The report calculated that to achieve a self-sufficient supply chain, countries competing with China would need to spend $78.2 billion on batteries, $60.4 billion on components and $13.5 billion on mining of lithium, nickel and cobalt, as well as $12.1 billion on refining of those materials, FT said.
Goldman forecast that the US market share of the Korean battery makers would soar to about 55% in three years, from 11% in 2021, FT said.
For now, China dominates battery production, including the mining and refining of raw materials.
The analysts said this dominance could be unwound by protectionist policies in Europe and the United States, coupled with alternative battery chemistries that require fewer critical minerals from China, FT reported.
Goldman Sachs did not immediately respond to a Reuters request for comment.
Graphite poised to do a lithium
Originally posted on Mining.com
By Frik Els
Andy Miller, COO Benchmark Mineral Intelligence presenting at BenchmarkWeek2022 in Los Angeles.
Pressure on carmakers in the EV battery supply chain is only building.
Original equipment manufacturers (OEMs) faced with an 8-fold increase in lithium prices, convulsions on the nickel market and ever-present worries about cobalt supply from the Congo, are being forced to look downstream to secure supply for their ambitious expansion plans.
Andy Miller, chief operating officer of Benchmark Mineral Intelligence, told an annual industry gathering in Los Angeles last week that soaring lithium prices and LME nickel market turmoil are signs of the huge momentum that is building in the battery supply chain.
“The events of the past 12 months are just the warning signs of what is to come across the raw material markets,” Miller said.
“All of this is being compounded by what is happening now at the policy level.
“It is no exaggeration to say that there is no bigger regulatory milestone for Western electric car markets than the Inflation Reduction Act, which has far-reaching consequences for all aspects of the energy transition.
“The impact goes well beyond US markets and will shape global trade as the EV supply chain is being built out,” he said.
Trading at a fraction of the prices of nickel, cobalt and high-purity manganese, anode material graphite is an often overlooked part of the EV supply chain.
In contrast to ternary cathode materials, graphite prices have drifted lower this year thanks to weakness in the steel industry, although there have been wide disparities between grades.
According to Benchmark’s Flake Graphite Price Assessment for October, China FOB 94-95% purity -100 Mesh sizes are up 31% over the past year, last trading at $765 a tonne while +100 Mesh prices have hardly moved over the same period to exchange hands for $890 a tonne. Benchmark also tracks the price of value added products such as uncoated spherical graphite (99.95%, 15 micron) which has risen by 10% in 2022 to average $3,065 a tonne.
Source: Via BenchmarkWeek2022
Both the mined and synthetic graphite market is at a turning point, said Miller.
Batteries became more than 50% of the cobalt market back in 2016 and the same happened for lithium in 2018, Miller said, and according to Benchmark analysis next year lithium-ion batteries will overtake the steel industry as the number one source of demand for graphite.
Benchmark sees demand for graphite over the next decade growing at an annual compound rate of 10.5% but supply will lag, expanding at only 5.7% per annum, despite a trend of supply diversification, particularly new mining projects coming in stream in Africa.
This year refractories and foundries will still dominate demand, but by 2025, the battery industry is set to consume two thirds of the world’s flake graphite, increasing to 79% in 2030, according to Benchmark’s Natural Flake Graphite Forecast.
Source: Via BenchmarkWeek2022
Weighty matter
The battery can make up 20% to 25% of the total cost of an EV and as the overall cost of batteries has come down, the bill of materials now equals roughly 70% of the cost of a cell.
And while the cathode is the most expensive component responsible for 50% of materials cost in a cell and another roughly 25% for other materials including separators, casings and electrolytes, by weight graphite constitutes 45% or more of the cell.
Miller told the BenchmarkWeek2022 conference held in person for the first time post-pandemic, given the tonnage of graphite required, why the industry has not seen the surge and prices or market disruptions in anode feedstock similar to that experienced in cathode material market:
“The fundamental reason is that graphite is starting from a much larger supply base and batteries have not yet become the dominant force.
“Up to now this flexible capacity has allowed the industry to respond pretty quickly to surges in demand.
“Through the mid-2020s we see an increasingly finely balanced graphite market, but if you look towards the end of the decade both synthetic and natural graphite face serious structural issues and significant supply deficit.”
Grafting onto graphite
Via BenchmarkWeek2022
Miller says given the long lead times for the extractive side of the graphite industry, to supply the coming deficit, the industry will have to start developing new mines ”today” and points out that most of the new mines will not be coming online at the shallow end of the cost curve.
At the average size of graphite mines of 56,000 tonnes per annum, the industry needs some 97 new mines and 52 new synthetic plants (average 57ktpa) to meet 2035 demand.
That’s not taking into account recycling, but the secondary market in graphite is not predicted to play a major role through this time frame given its lower value compared to cathode materials. The number of mines needed is also more than that for lithium (59), cobalt (62) and nickel (72).
Benchmark estimates global this year’s flake graphite supply of roughly 1.5m tonnes will double by 2027 driven by new mines and expansions in Tanzania, Mozambique, Madagascar and Namibia.
OEMs swimming upstream
Miller says auto manufacturers are waking up to the importance of the anode side of the supply chain.
Via BenchmarkWeek2022
Over the last 12 months a number of OEMs have made direct purchase agreements with graphite developers, including Tesla and Syrah Resources which operates the Balama mine in Mozambique, General Motors and Korea’s Posco (2024 onwards) and both Daimler and Stellantis with Talga Group, owners of the Vittangi graphite mine in Sweden. Quebec-based Nouveau Monde Graphite earlier this month signed a partnership and off-take agreement with Panasonic, Tesla’s main supplier.
Chinese control
China has a firm grip on the lithium, cobalt and nickel supply chain, but its dominance in graphite is even greater.
China boasts around 30 of the 45 mines in operation around the world. The country supplies 64% of the world’s natural graphite and 55% of the world’s needle coke, a derivative of crude oil and coal tar and a precursor for synthetic graphite anodes.
Overall, synthetic graphite output is 68% Chinese while the country supplies a full 90% of the world’s anodes.
For uncoated spherical graphite which (99.95% purity), China is in charge of 100% of the world’s production with the bulk coming from Heilongjiang province. Even ten years out some four-fifths of this market will still be controlled by China, according to Benchmark forecasts.
While synthetic anodes are currently more sought after by carmakers because they improve charging times and battery life, natural graphite-heavy anodes will find favour in future, boosted also by the increasing addition of silicon to anode chemistries.
Benchmark forecasts natural graphite anode demand to grow by more than 400% by the end of the decade versus 170% for synthetic. Alternative anodes including silicon, lithium titanate (LTO) and mesocarbon microbeads (MCMB) will gain market share but graphite will still make up more than 80% of the market by 2040, according to Benchmark.
Synthetic emissions
Demand for natural graphite is set to overtake synthetic before the end of the decade because of carmakers’ worries about the environmental impact of the latter, the need to cut costs and to diversify the supply base away from China.
On a CO2 per kilogram of graphite anode basis, natural graphite production in China is half that of synthetic production across the country. And when compared to synthetic graphite production in Inner Mongolia, which relies almost exclusively on coal fired power plants, the emissions rise to more than three times as much as natural graphite.
Via BenchmarkWeek2022
Underinvestment by the oil industry also means no new needle coke plants have been built for decades in the US. At the same time, China has become a net importer of graphite to feed its battery supply chain and Benchmark notes greater involvement by Beijing, local governments and state-owned enterprises in the industry, including policy and financial incentives for new mines and plants.
Canada's battery supply credibility jumps as multi-billion announcements keep coming
Originally posted on Nationalpost.com
Prime Minister Justin Trudeau arrives with Rio Tinto chief executive Jakob Stausholm, left, and Industry Minister Francois-Philippe Champagne, right, for a tour of a new pilot project for a blue smelting facility at the Rio Tinto plant, on Tuesday, Oct. 11, 2022 in Sorel, Que. PHOTO BY RYAN REMIORZ /The Canadian Press
OTTAWA — Federal Innovation Minister Francois-Philippe Champagne is selling Canada’s battery-supply chain prowess in Asia again this week, but this time he has a new boast in his back pocket.
Research firm BloombergNEF pushed Canada’s position in its annual global ranking of battery-producing countries ahead of everyone else but China.
“That’s something I’m going to use very much on my trip in Asia, to say we have what Asia needs,” Champagne said.
The survey ranks 30 countries with a significant presence in the industry, be it in the mining of raw materials to the production of batteries and their component parts.
The first version in 2020 ranked Canada fourth, and in 2021 fifth, after mining outputs fell and regulatory hurdles mounted.
But Canada has announced more than $15 billion in investments over the past 10 months in areas ranging from critical mineral mining and processing to battery component manufacturing, electric vehicle production and the country’s first gigafactory.
That helped Canada climb past Sweden, Germany and the United States, even with the latter’s massive investments under the Inflation Reduction Act.
“I think this is a home run for Canada in the sense that the vision was really to build an ecosystem from mine to recycling, and now it’s taking shape and what we’re doing now is to optimize it,” Champagne said.
His trips this week to Japan and South Korea, along with next week’s planned stops in Germany, are in that vein. He has already met with key industry players in those countries multiple times both in Canada and abroad, but he says he’s focused on consolidating those relationships and continuing to make Canada’s case as a presence in the field.
The battery-supply chain has many links, starting with mining of the raw materials like lithium, nickel, aluminum and copper used to make batteries. Those minerals and metals are then refined so they can be used to make the components of battery cells — namely cathodes, anodes and electrolytes.
The components are then pulled together to make battery cells — which resemble the same alkaline non-rechargeable batteries most consumers are familiar with — and then gigafactories package those cells together in large numbers to make battery packs that run everything from laptops and cellphones to electric cars.
The BloombergNEF report looks at all of those supply chain parts, as well as demand for the end product and environmental stewardship.
Canada gets among the highest marks on keeping the supply chain green, thanks in part to a generous supply of renewable energy but also to environmental regulations on mining. The BloombergNEF survey also credited Canada for its efforts to boost mining activity.
Canada is still lagging on battery cell and component manufacturing and domestic demand for electric vehicles, but there have been many announcements in the last year improving both.
Vic Fedeli, Ontario’s minister of economic development, told The Canadian Press following a trip to meet with industry stakeholders in Germany last month that one of Canada’s biggest selling points is its access to the raw materials needed to make batteries.
“They talk about our critical minerals and that’s when we know we’ve got their genuine interest because there’s such a finite amount of active critical mineral producers outside of China,” he said. “We really have a captive audience.”
While Canada is not the biggest producer of any of the main metals and minerals needed for batteries, it is one of the few places in the world capable of producing all of them.
Canada and its allies are also trying to prevent China from using its dominance in the battery supply chain industry to throw its weight around in global politics. They have likened it to Europe being too reliant on Russia for gas.
Having started investing in the sphere more than a decade ago, China is now home to three-quarters of all battery cell manufacturing capacity and 90 per cent of anode and electrolyte production.
Its raw mineral production isn’t always the highest, but it has invested heavily in mines in other countries, including in Canada, to bring those products to China for refining and use in manufacturing. The U.S. Geological Survey said China produced about four per cent of the world’s nickel last year but refined more than two-thirds of it.
It mined about 14 per cent of the lithium produced in 2021 but refined 59 per cent.
Canada is starting to take steps to limit China’s influence within the domestic supply chain. Earlier this month, Champagne said Canada will limit the involvement of foreign-owned state companies in the critical mineral sector, and a week later ordered three Chinese companies to sell their interests in small Canadian firms.
But there are many more, including the only currently operating lithium mine in Canada. The Tanco mine in Manitoba is owned by China-based Sinomine Resource Group.
Champagne wouldn’t say what other orders will come, but did hint at additional announcements.
“I’ll be like a hawk looking at these transactions to make sure that we protect the national and economic security of Canadians,” he said.
U.S. military weighs funding mining projects in Canada amid rivalry with China
Originally posted on Cbc.ca
By Alexander Panetta
The Pentagon, the U.S. military headquarters in Washington, is being asked to fund civilian projects to build more reliable supply chains of critical minerals that are vital in everything from products like electronics, cars and batteries, to weapons. Canadian companies are entitled to apply. (Jason Reed/Reuters)
The United States military has been quietly soliciting applications for Canadian mining projects that want American public funding through a major national security initiative.
It's part of an increasingly urgent priority of the U.S. government: lessening dependence on China for critical minerals that are vital in everything from civilian goods such as electronics, cars and batteries, to weapons.
It illustrates how Canadian mining is becoming the nexus of a colossal geopolitical struggle. Ottawa just pushed Chinese state-owned companies out of the sector, and the U.S. is now considering moving public funding in.
The American military has a new pot of money at its disposal to help private companies inaugurate new mining projects; it's for funding feasibility studies, plant renovations, battery-recycling and worker training.
President Joe Biden invoked the 1950 Defense Production Act to expand the domestic mining sector, and the military received hundreds of millions of dollars to implement it.
This whirlwind of activity was prompted by a White House study last year warning that dependence on certain foreign-made products represents a national security risk to the U.S., and it cited semiconductors, batteries, medicines and 53 types of minerals.
U.S. President Joe Biden, shown speaking at a virtual roundtable in Washington in February, invoked the U.S. Defense Production Act in March to fund critical minerals projects needed for such technologies as electric vehicles. (Kevin Lamarque/Reuters)
An official from the U.S. Department of Defence this week provided a briefing on the program at a cross-border conference, and he made one thing clear about the funding: Canadians qualify.
That's because Canada has, for decades, belonged to the U.S. military industrial base and is every bit as entitled to the cash as American mining projects.
"It's really quite simple. It's a matter of law," said Matthew Zolnowski, a portfolio manager for the Defense Production Act program, speaking to a gathering of the Canada-United States Law Institute in Washington, D.C.
"So an investment in Alberta or Quebec or Nova Scotia would be no different than if it was in Nebraska or anywhere else in the United States. As a matter of law."
Canadian government provides list of 70 projects
Zolnowski said the U.S. is actively reaching out to companies to explain the process, as many have no relationship with the U.S. government and might not realize how it works.
"We are actively engaging those firms," he said, describing a flurry of recent activity by quoting an old movie line: "It's a duck on a pond. It looks quiet on the surface, but there's a lot happening."
The Canadian government has been active, too. Canadian officials say they've already provided the U.S. with a list of 70 projects that could warrant U.S. funding.
Both countries describe this as a generational initiative still in its early stages: Canada, for now, is still a bit player in producing these minerals, which include lithium, cobalt and manganese.
But one Canadian official said this can change. Jeff Labonté, assistant deputy minister at Natural Resources Canada, told the conference that Western democracies are now engaged in industrial policy in a way they haven't been for decades.
"We have this resource potential.... We also have a huge capacity," he said, touting 200 mines and 10,000 potential products in the exploration phase.
"We have a skill set in this area. We have capital markets, we have engineering expertise, we have companies that operate around the country and around the world."
Canada is also providing billions of dollars in public funds to the sector over the coming years through federal and provincial programs.
If it opens on time next March, the mine in La Corne, Que., will be one of the only functional lithium mines in North America. Electric vehicles are hugely reliant on minerals like lithium. (Sayona Québec )
What's driving this sudden minerals rush?
The transition to electric cars is a key driver of this challenge. They're hugely reliant on minerals like lithium, and current production is not close to meeting projected demands.
Making matters more complicated is China's dominance of the market; it controls two-thirds of the world's lithium processing capacity, for example.
Beijing has already revealed a willingness to cut off rivals from mineral exports, as it did a few years ago amid a fishing dispute with Japan.
The U.S. has, more recently, suspended semiconductor exports to China in an emerging digital cold war in which Canada is increasingly involved.
A worker in Inner Mongolia stokes pots of lanthanum in 2010, the year China cut off exports to Japan in a dispute over sea access. China dominates the critical minerals sector. (David Gray/Reuters)
In his talk, Zolnowski said countries spent decades leaving themselves in this vulnerable position; resolving it won't happen overnight.
He said the U.S. government has a four-part strategy for this.
Part 1 is to stimulate domestic demand for these goods by designing new sustainability initiatives around these materials.
Part 2 is stimulating supply by funding new production and recycling, while Part 3 is building stockpiles. The final component involves working with allies.
Zolnowski noted that back in 1984, Robert Gates, at the time a U.S. intelligence official who went on to become secretary of defence to two presidents, articulated his fear in a speech that foreign government-funded companies would come to dominate the industry.
This worries the Pentagon for security reasons, both economic and military. Zolnowski called these minerals the building blocks of a thriving economy.
Prime Minister Justin Trudeau, left, speaks with a worker during a tour of Motrec International, a heavy-duty electric vehicle production facility in Sherbrooke, Que., in July. (Graham Hughes/The Canadian Press)
And in times of war, he said, industrialized nations that lack secure and reliable access to these materials have suffered mightily: "[They] have suffered significant performance tradeoffs, which contributed to their defeat."
He said civilian goods will dominate the market, as well as receiving the lion's share of Pentagon funding. Indeed, the language of the Defense Production Act stipulates that funds can be used for non-military purposes, including the U.S.'s general economic well-being.
Pentagon's main role: Building market confidence?
Zolnowski said the U.S. is looking primarily at offering grants, not loans, and it's willing to fund projects at various phases of implementation, as it views this as a long-term project.
One partner at an investment firm present at the conference said the Pentagon's role is not to become a major investor.
What the private sector wants, he said, is help with confidence-building: Once you demonstrate that a project has the Pentagon's imprimatur, he said, it's easier to reassure investors this is a safe bet.
One attendee said there are still flaws to iron out in the program design of Canada's own critical minerals strategy, including its 30 per cent tax credit.
Jonathan Garbutt, a Calgary-based tax lawyer, cited industry estimates that lithium extracts from brine deposits in Western Canada could produce hundreds of thousands of tonnes per year, but, under the current language of the Income Tax Act, the credit wouldn't apply to those extracts.
Another speaker at the conference noted that this new conversation about cross-border co-operation carries historical echoes.
Franklin D. Roosevelt, second from left, Winston Churchill and William Lyon Mackenzie King — leaders of the United States, Britain and Canada, respectively — are shown at the Quebec Conference in September 1944. Back then, Canada-U.S. military co-operation was built around aluminum. (The Canadian Press)
International trade lawyer Lawrence Herman, who is based in Toronto, noted that the precursor to the countries' current military-industrial partnership was a 1940 agreement between the U.S. and Canadian leaders.
Back then, American funding discreetly helped turn Quebec aluminum into a global powerhouse.
Since then, Quebec aluminum has had mostly civilian uses. It also helped the U.S. build its arsenal for the Second World War.
Canada was heavily involved enough in that effort that Quebec became the site of the wartime allied leaders' conference.
Global EV Sales for 2022 H1
Originally posted on Ev-volumes.com/
By Roland Irle, EV-Volumes
Global EV sales continue strong. A total of 4,3 million new BEVs and PHEVs were delivered during the first half of 2022, an increase of +62 % compared to 2021 H1. The regional growth pattern is shifting, though. Following 2 years of steep sales increases in Europe, EVs gained only +9 % over 2021 H1 there. Weak overall vehicle markets and persistent component shortages have taken their toll, exacerbated by the war in Ukraine. EV sales in USA and Canada increased by 49 % for H1 year-on-year, despite a weak overall light vehicle market which plunged by 17 % during H1 y/y.
China NEV sales defied all challenges the country is facing otherwise (real estate crisis, Covid lock-downs) and increased by a staggering 113 % for H1. BYD more than quadrupled sales to 641 000 units, making it the #1 in the global sales ranking, if their 315 000 PHEV sales are included. Counting BEVs only, Tesla still leads by a wide margin with 565 000 units delivered in H1.
PHEVs stood for 27 % of global Plug-in sales in 2022 H1 compared to 29 % in 2021. While their sales volumes still increase, their share in the PEV mix is in decline, facing headwinds from incentive cuts and improving BEV offers. Sales growth is increasingly depending on the degree of electrification. While BEVs grew by +75 % and PHEVs by +37 %, non-chargeable Full Hybrids grew by +14 % and Mild Hybrids backed by -7 % y-o-y H1. ICE-only vehicle sales declined by -16 %. Total global light vehicle sales were down 8 % y/y for H1-2022.
Rapid EV adoption in weak auto markets has boosted EV shares further. BEVs (8,2 %) and PHEVs (3,1 %) stood for 11,3 % of global light vehicle sales at H1 close, compared to 6,3 % in 2021 H1. Norway had the highest market share of EVs in H1 (BEV 69 % + PHEV 8 %), China had 21 %, Europe 18 % and USA 6,5 %. The fastest growing markets were India with 20 700 units BEV & PHEV for H1, +273 % and New Zealand with 8 300 units, +260 %.
For the full year of 2022, we expect sales of 10,6 million EVs, a growth of 57 % over 2021, with BEVs reaching 8 million units and PHEVs 2,6 million units. By the end of 2022 we expect nearly 27 million EVs in operation, counting light vehicles, 70 % are BEVs and 30 % PHEVs. Sales of Fuel Cell Vehicles (FCEV) in the light vehicle sector have declined by -9 % so far and are below 20 000 units annually. Current sales are from 5 vehicle models and most sales are in South Korea and USA. We estimate their current population to ca 55 000 units.
High EV Growth in Weak Vehicle Markets
Global light vehicle markets are contracting again. Following a brief recovery in 2020 H2 and 2021 H1, 2022 H1 sales were 8,1 % lower than last year. We expect small gains for H2 as numbers compare to the depressed sales of 2021 H2. 2022 auto sales in most mature markets stayed 20-30 % below the 2015 - 2019 average, so far.
China is among the few exceptions, but with extreme volatility: a +24 % increase y/y in February was followed by a -45 % crash during the April lock-downs and a +30 % boom in June and July, amid swift Government support.
Western auto sales are facing a double dip, post-Covid, with supply constraints soon to be joined by demand constraints as central banks try to combat inflation by interest rate hikes. The result is, much likely, a further delay in market recovery.
EV sales held up well in this environment: while global light vehicle sales lost -8,1 %, BEVs and PHEVs increased by +62 % for H1. The relative weakness in Europe's EV growth relates to the EV boom in 2020 / 2021 and the repercussion from the war in Ukraine.
New all-time-highs in EV sales will be common also during the 2nd half, but growth rates will be somewhat lower as the low-base-effect diminishes.
BYD Leads - Including Plug-in Hybrids
Robust increases of EV sales enabled nearly all OEM to grow their sales in 2022-H1. Global EV deliveries increased by +62 % y/y in total; OEMs with higher growth have increased their share in the EV sector.
BYD sold over 4 times as many BEVs+PHEVs compared to 2021 H1, by boosting sales of existing models, successfully introducing new models and by entirely focusing production and sales on BEVs and PHEVs. Non-chargeable variants were phased out, ending in April this year. BYD is now the largest maker of PHEVs and moved from rank #3 in 2021 to #1 for BEVs & PHEVs combined.
Tesla leads global sales of BEVs by a large margin, with a share of 18 % in all BEVs sold worldwide. H1 growth was 46 %, less than for the sector, but from a high base.
The VW Group stayed flat compared to last year; with all its brand following this pattern we can assume production related issues. BEVs gained, while PHEVs lost.
GM increased by just 15 % as the Wuling Mini-EV is facing more competition and most future, high volume EV entries still are in the pipeline.
Hyundai and Kia launched at least 9 new and revised EVs during the last 18 months, among them the Ioniq 5, the Kia EV6, the Kia Niro and 3 EVs from Genesis. Their global growth outperforms the sector and is the more impressive as the companies EV presence in China is fading.
Increases of Stellantis were below the global EV sector growth, with high exposure to slow growing Europe and low sales in the strongest market, China. Excluding China, their sector share has risen from 11 % in H1-2021 to 12,7 % this year.
Other OEMs of European and/or Japanese origin did not keep up with the sector growth as they were held back by disturbed supply chains and/or underestimated EV demand during weakening auto markets. Toyota and Jaguar Land Rover delivered fewer EVs compared to last year.
Most Chinese brands show triple digit growth of NEV sales in their booming home-market. Exports accounted for only 3,6 % of their global volume, not including China exports by e.g Tesla, Polestar, BMW iX3 and other transplants. Including these, exports accounted for 8,4 % of China NEV production in H1 this year.
Federal government moves to cut China out of Canadian critical mineral industry
Originally posted on Cbc.ca
By Mia Rabson
Minister of Innovation, Science and Industry François-Philippe Champagne rises during question period in the House of Commons in Ottawa on Thursday. (Justin Tang/The Canadian Press)
After a national security review, Innovation Minister François-Philippe Champagne is ordering three Chinese resource companies to sell their interests in Canadian critical mineral firms.
Champagne's order comes less than a week after he said Canada would be limiting the involvement of foreign state-owned companies in the industry.
Critical minerals and metals, such as lithium, cadmium, nickel and cobalt, are essential components of everything from wind turbines and electric cars to laptops, solar panels and rechargeable batteries.
China is the dominant player in critical mineral refining and processing, and in the manufacturing supply chain of battery cell components.
But China does not produce a lot of the minerals, and has instead invested heavily in overseas mines in places like Canada to acquire the raw materials it needs.
Canada and its allies are desperately trying to upend China's dominance in the field and create a supply chain that relies on what are deemed more stable and reliable partners.
An electric vehicle being charged in Ottawa in July. Manufacturing EV batteries requires certain so-called critical minerals, few of which are found in North America. (Sean Kilpatrick/The Canadian Press)
"While Canada continues to welcome foreign direct investment, we will act decisively when investments threaten our national security and our critical minerals supply chains, both at home and abroad," Champagne said in a written statement late Wednesday.
The Investment Canada Act subjects foreign investments to review for national security concerns and Champagne said critical mineral investments get "enhanced scrutiny."
He said a "multi-step national security review process" by national security and intelligence agencies concluded three companies must divest their holdings in Canadian critical mineral companies.
The order requires Sinomine (Hong Kong) Rare Metals Resources to sell its investment in Vancouver-based Power Metals Corp., which has exploration projects for lithium, cesium and tantalum in northern Ontario.
Chengze Lithium International Ltd. is required to divest its interests in Lithium Chile Inc., a company headquartered in Calgary with more than a dozen lithium projects underway in Chile.
And Zangge Mining Investment is ordered to sell its investment in Ultra Lithium Inc., a Vancouver-based resource development firm with lithium and gold projects in both Canada and Argentina.
Canada and the U.S. have both identified dozens of minerals and metals they deem essential to their future economic success.
They point to the instability created by Europe's reliance on Russia for oil and gas after the Russian invasion in Ukraine last winter, and growing tensions with China as reasons to ensure supply chains rest mostly in the hands of friends and allies.
In June, U.S. Treasury Secretary Janet Yellen referred to it as "friend-shoring" during a trip to Ottawa.
"So friend-shoring is the idea that countries that espouse a common set of values about international trade, conduct in the global economy, should trade and get the benefits of trade so we have multiple sources of supply and are not reliant excessively on sourcing critical goods from countries where we have geopolitical concerns," Yellen said.
The new rules for critical mineral investments announced by Champagne last week mean investments by state-owned firms will only be approved on "an exceptional basis" and will apply to investments of any size, from small stakes all the way to outright takeovers.
It will affect everything from exploration and development to mining, refining and processing.
Federal Natural Resources Minister looks to speed up Canadian critical minerals production
Originally posted on Northernontariobusiness.com
By Ian Ross
Federal Natural Resources Minister Jonathan Wilkinson speaks at the Canadian Club of Toronto, Oct. 25. (Supplied)
With a global critical minerals supply gap coming, federal Natural Resources Minister Jonathan Wilkinson admits Ottawa has some work to do toward expediting approvals to put more critical minerals mining projects into production sooner.
In his Oct. 25 remarks to Canadian Club of Toronto, Wilkinson said Ottawa is looking to get on the same page with the provinces and territories in working smarter in advancing energy and natural resource projects along in a timely manner.
Wilkinson said there’s a clear need to find ways to develop projects “more rapidly than what we have been able to in the past.”
The bulk of his speech had much to do with Canada’s clean energy transition and fulfilling this country’s obligation to help its European allies struggling with an energy crisis following the cutoff of Russian oil and gas stemming from conflict in Ukraine.
But during the question-and-answer period, Wilkinson said on the domestic front, Ottawa faces interjurisdictional challenges. Each province has its own regulatory and permitting process for natural resource projects. Often there are situations where these federal and provincial processes work consecutively, rather than concurrently.
He cited the mining industry as a particular sector where Ottawa and the provinces must find ways to work more collaboratively and efficiently.
“In Canada, it typically takes us at least about 12 years to go from beginning to mine opening. On the international stage, it’s actually worse — it’s 15 to 18 years.
“If it really takes us 12 years …. we will not have sufficient quantities of the minerals that we need to actually build the cars that are required to actually get rid of the emissions in the transportation sector.
“So we have to figure out how to do things better and go faster.”
Other countries face similar challenges as Canada, he said.
Wilkinson said U.S. Energy Secretary Jennifer Granholm often mentions that the White House looks to Canada’s environmental assessment processes as a model “because we can get mines permitted and they cannot.”
Energy and critical minerals projects need to be fast-tracked, he said, but also move at a pace that doesn’t compromise the government’s climate change principles and abides by the federal Impact Assessment Act passed in 2019.
“How can we actually find ways to not cut corners from environmental perspective, not cut corners in terms of discharging our obligations to Indigenous peoples, but do things far more efficiently to get to an end state that actually allows us to make decisions on projects.”
This issue involves a greater interdepartmental discussion in Ottawa as to how to optimize their processes, he said.
Accelerating project development and figuring out how to streamline regulatory processes was outlined in the federal critical minerals strategy released last March and mentioned by Wilkinson at the Prospectors and Developers Association of Canada mining convention last June.
One way Ottawa hopes to align their regulatory and permitting regimes with the provinces and territories is through Regional Energy and Resource Tables.
Ontario joined this initiative yesterday along with British Columbia, Newfoundland-Labrador, Manitoba, Yukon, Northwest Territories, Prince Edward Island, Nova Scotia and New Brunswick.
Through this partnership, Wilkinson said, each provincial or territory will identify three to five areas of economic growth opportunities to transform regional industries and advance emerging ones. These collaborations are very much tied to Ottawa’s clean energy transition movement.
The Biden Administration has jumped on the green energy bandwagon in a big way with the passage of the Inflation Reduction Act this year. It provides a slew of subsidiaries and tax incentives designed to boost U.S. and North American production of critical minerals and establish a safe and secure domestic supply chain for technology and electric vehicle manufacturers.
Wilkinson said Canada welcomes this new legislation and hinted Ottawa will respond in a similar fashion to ensure this country remains a competitive jurisdiction to attract investment. The act has created an “unlevel playing field” that Canada must somehow level out, especially when it comes to placing mineral processing and battery manufacturing plants.
He’s urging his U.S. counterparts that this cannot become a game of legislative one-upmanship. “At the end of day, we need to be working on this together.”
But Wilkinson also reminded the audience that Canada has considerable leverage in its dialogue with the U.S.
“The Americans need our critical minerals.”
Talk also turned toward Indigenous equity in resource projects. It's a movement that’s started in the Western Canadian provinces as a means to achieve economic reconciliation, a phrase, Wilkinson said, not often mentioned in the broader national conversation.
Ontario’s electricity sector and the oil and gas industries are Canadian leaders in striking successful 50/50 partnership with Indigenous communities on new projects. The same kind of leadership needs to occur on the critical minerals front and in other resource sectors, he said.
Wilkinson said when mining companies enter a First Nation’s traditional territory, propose a big mine and promise a community only a handful of jobs and a new community centre, “it’s no wonder many folks would say, why would we agree to that?”
There must be pathways for Indigenous communities to have a seat at the table to derive a project’s long-term benefit and have a voice on how the project gets built.
“It changes the nature of the conversation.”
Ottawa is looking at financial tools to enable First Nation communities to participate in these projects. It could take the form of loan or loan guarantees.
“Part of it is about reconciliation, part of it is ensuring good projects can go ahead in a manner that is actually consistent with Indigenous perspectives. I think it’s a critically important issue.”